Staff profile
Professor Martin Bryce
Professor

| Affiliation | Telephone |
|---|---|
| Professor in the Department of Chemistry | +44 (0) 191 33 42018 |
Biography
Martin Bryce was born near Birmingham, UK and graduated from Wolverhampton Polytechnic (B.Sc. 1st class). He obtained a D.Phil. from York University in 1978 for work on synthetic methodology for sulfur and selenium heterocycles under the guidance of John Vernon and Peter Hanson. Following postdoctoral positions at the University of British Columbia,Vancouver (in Larry Weiler’s group) and the University of Bristol (in Roger Alder’s group) he joined Durham University. He was promoted to Professor of Chemistry at Durham in 1995. He is the recipient of a Ciba-Geigy Award for academic collaboration in Europe (1990), the Royal Society of Chemistry Bader Award (1992), the Royal Society of Chemistry Interdisciplinary Award (1992), the Nuffield Foundation Science Research Fellowship (1993), the University of Durham Sir Derman Christopherson Fellowship (1995) and the Royal Society of Chemistry Heterocyclic Chemistry Award (2002). Martin has held Visiting Scientist positions at the University of California at Santa Barbara, and the University of Copenhagen. He was a Troisième Cycle Lecturer in Switzerland in 2008 and a Tarrant Visiting Professor at the University of Florida, Gainesville in 2013. He was the co-director of the Durham University Centre for Molecular and Nanoscale Electronics (1990-2018). He was the Scientific Editor of the Royal Society of Chemistry's Journal of Materials Chemistry (1995-2000). Martin coordinated the EC FP7 Marie Curie ITNs “Fundamentals of Molecular Electronic Assemblies” (FUNMOLS) (2008-2012) and “Molecular-Scale Electronics” (MOLESCO) (2014-2017) comprising 10 European partner laboratories.
Research Interests
Our work is rooted in new synthetic chemistry. Current projects include:
Materials for Optoelectronic Applications
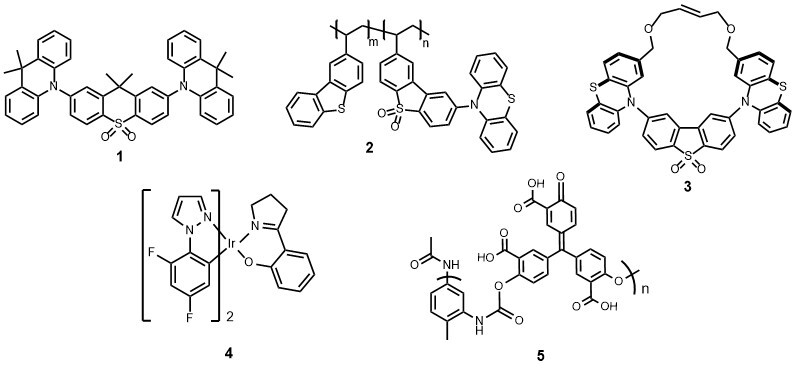
The ability to control the optoelectronic properties of conjugated molecular and polymeric systems is a fascinating aspect in the design of new materials for organic light-emitting devices (OLEDs) and displays that offer bright colours and high stabilty. Deep-blue fluorescent and phosphorescent emitters and white-light devices (WOLEDs) are of particular current interest. Representative work concerns small molecules and polymers that possess thermaly activated delayed fluorescence (TADF), such as structures 1-3,1-3 and room temperature organic phosphorescence.4 We have reported new emitters and OLEDs based on phosphorescent cyclometallated Ir complexes.5,6 This is very interdisciplinary work, some projects involving close collaboration with colleagues in the Department of Physics in Durham (Professors Andy Monkman and Fernando Dias), and with industrial sponsors who are commercialising some of these materials for display technologies and lighting applications.
We are also studying luminescent organic and organometallic materials for sensing explosives, monitoring volatile organic compounds and for anti-conterfeiting and data encryption,7 for example iridium complex 48 and polyurethane derivative 5.9 These projects are in collaboration with Northeast Normal University, Changchun (Professor Dongxia Zhu).
Molecular and Nanoscale Electronics
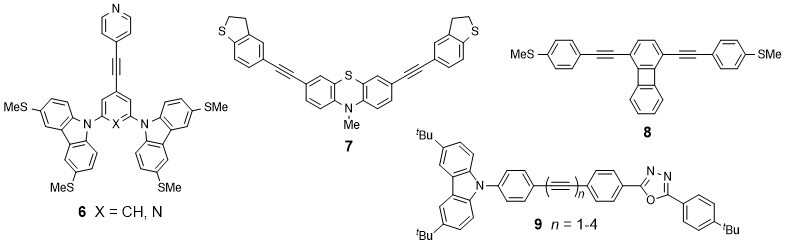
Molecular electronics is attracting great attention due to potential applications in future computing technology, energy harvesting and storage, and related fields. A wide range of “molecular wires“, including structures 6-8, have been synthesised recently in our group.10-12 Their fundamenal optoelectronic, electrical and thermoelectrical properties are being developed in partnership with academic and industrial laboratories in Europe (notably IMDEA-Madrid, IBM-Zurich and Lancaster University) and China (Xiamen University) using specialised experimental techniques and theoretical expertise. Assembly and characterisation of molecules in metal | molecule | metal junctions is a prominant aspect of this work.
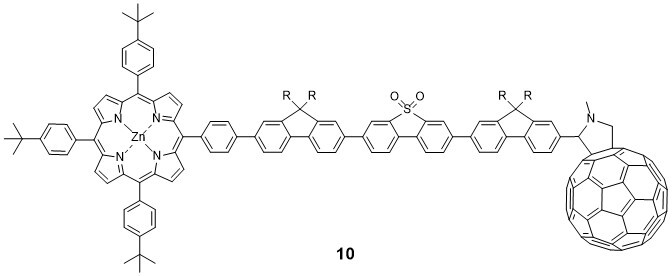
Related projects probe photoinduced charge transport through pi-conjugated wire-like molecules such as 913 and 1014 end-capped with electron donor and acceptor units.The techniques include cyclic voltammetry, spectroelectrochemistry, steady-state and time-resolved photolysis, X-ray crystallography, ESR spectroscopy and theoretical calculations.
Molecules for Photodynamic and Photothermal Therapy
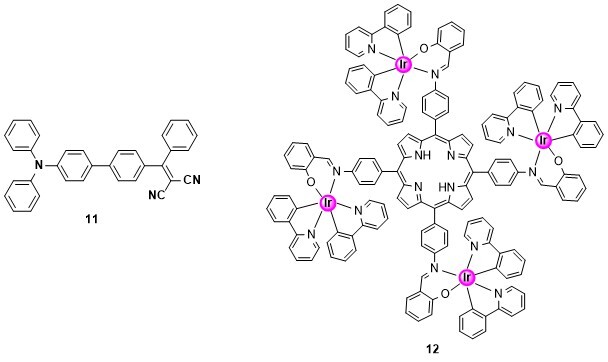
In collaboration with laboratories in Changchun we have designed, synthesised and evaluated new molecules for photodynamic and photothermal therapy. The work is focused on red and near-infrared emitting species with high biocompatibility. In vitro and in vivo experiments have established that molecules 1115 and 1216 encapsulated in nanoparticles exhibit potent cytotoxicity towards cancer cells and effectively inhibit tumour growth with high therapeutic efficacy.
References
- Stachelek, P. et al, ACS Appl. Mater. Interfaces 2019, 30, 27125-27133. DOI: 10.1021/acsami.9b06364.
- Li, C. et al, Adv. Opt. Mater. 2017, 5, 1700435. DOI: 10.1002/adom.201700435.
- Hempe, M. et al, J. Org. Chem. 2021, 86, 429-445. DOI: 10.1021/acs.joc.0c02174.
- Chen, C. et al, Angew. Chem., Int. Ed. 2018, 57, 16407-16411. DOI: 10.1002/anie.201809945.
- Du, M. et al, Adv. Mater., 2016, 28, 5963-5968. DOI: 10.1002/adma.201600451.
- Congrave, D. G. et al, Inorg. Chem. 2018, 57, 12836-12849. DOI: 10.1021/acs.inorgchem.8b02034.
- Jiang, Y. et al, Chem. Commun. 2017, 53, 3022-3025. DOI: 10.1039/c7cc00769h.
- Che, W. et al, Chem. Commun. 2018, 54, 1730-1733. DOI: 10.1039/c7cc08832a.
- Jiang, N. et al, Chem. Mater. 2020, 32, 5776-5784. DOI: 10.1021/acs.chemmater.0c01620.
- O’Driscoll, L. J. et al, Angew. Chem. Int. Ed. 2020, 59, 882-889. DOI 10.1002/anie.201911652.
- Liu, J. et al, Angew. Chem. Int. Ed. 2017, 56, 13061-13065. DOI: 10.1002/anie.201707710.
- Chen, H. et al, Nanoscale 2020, 12, 15150-15156. DOI: 10.1039/d0nr03303k.
- Zieleniewska, A. et al, J. Am. Chem. Soc. 2020, 142, 18769-18781. DOI: 10.1021/jacs.0c04003.
- Yzambart, G. et al, J. Phys. Chem. C 2017, 121, 13557-13569. DOI: 10.1021/acs.jpcc.7b03889.
- Zhang, L. et al, Chem. Sci. 2020, 11, 2369-2374. DOI: 10.1039/c9sc06310b.
-
Zhang, L. et al, Chem. Sci. 2021, 12, 5918–5925. DOI: 10.1039/d1sc00126d.
Research interests
- Molecular Electronics
- Functional Materials
- Organic Synthesis
Publications
Conference Paper
- Oligo(fluorenyl)pyridine ligands and their iridium complexes: Synthesis, photophysical properties and electrophosphorescent devicesTavasli, M., Bettington, S., Bryce, M., Monkman, A., Al Attar, H., Dias, F., & King, S. (2005). Oligo(fluorenyl)pyridine ligands and their iridium complexes: Synthesis, photophysical properties and electrophosphorescent devices. In Abstracts of Papers of the American Chemical Society (pp. U966-U966).
Journal Article
- A Metalloporphyrin Nanosystem Enables Non‐Invasive Visualization and Specific Treatment for Thrombosis and Ischemic StrokeWang, Z., Zhang, L., Wu, Z., Xiao, N., Zheng, W., Wang, Y., Xu, G., Zhu, D., Bryce, M. R., Ren, L., & Tang, B. Z. (2025). A Metalloporphyrin Nanosystem Enables Non‐Invasive Visualization and Specific Treatment for Thrombosis and Ischemic Stroke. Advanced Science, e15079. https://doi.org/10.1002/advs.202515079
- Unraveling the Role of Triplet-Triplet Annihilation and Photodegradation in Difluoroboron-Based Organic Laser Gain Materials.Kuila, S., Miranda-Salinas, H., Li, C., Pridmore, N. E., Bryce, M. R., Marian, C. M., & Monkman, A. P. (2025). Unraveling the Role of Triplet-Triplet Annihilation and Photodegradation in Difluoroboron-Based Organic Laser Gain Materials. Angewandte Chemie (International Ed. In English). Advance online publication, Article e202509535. https://doi.org/10.1002/anie.202509535
- A New Strategy Enabling Combined Fluorescence Imaging of Individual Tuberculous Granulomas and Precise Photothermal Therapy of Tuberculosis With Lesion‐ and Pathogen‐Targeting Capabilities of the NanoparticlesZhu, D., & Bryce, M. R. (2025). A New Strategy Enabling Combined Fluorescence Imaging of Individual Tuberculous Granulomas and Precise Photothermal Therapy of Tuberculosis With Lesion‐ and Pathogen‐Targeting Capabilities of the Nanoparticles. Aggregate, 6(8), Article e70110. https://doi.org/10.1002/agt2.70110
- Sonodynamic and Bioorthogonal Sonocatalytic Thrombotic Therapy Based on AIE Cationic Tetranuclear Ir(III) Complex Nanoplatform Guided by NIR‐Chemiluminescence ImagingWu, Z., Zhang, L., Wang, Z., Liu, S., Zhang, Q., Shi, C., Wang, Y., Xu, G., Zhu, D., Bryce, M. R., Ren, L., & Tang, B. Z. (2025). Sonodynamic and Bioorthogonal Sonocatalytic Thrombotic Therapy Based on AIE Cationic Tetranuclear Ir(III) Complex Nanoplatform Guided by NIR‐Chemiluminescence Imaging. Advanced Materials. Advance online publication, Article 2503599. https://doi.org/10.1002/adma.202503599
- Endogenous Near‐Infrared Chemiluminescence: Imaging‐Guided Non‐Invasive Thrombolysis and Anti‐Inflammation Based on a Heteronuclear Transition Metal ComplexWang, Z., Zhu, B., Nie, W., Zhang, L., Xiao, N., Zhang, Q., Wu, Z., Shi, C., Zhu, W., Liu, Q., Zhu, D., Bryce, M. R., Ren, L., & Tang, B. Z. (2025). Endogenous Near‐Infrared Chemiluminescence: Imaging‐Guided Non‐Invasive Thrombolysis and Anti‐Inflammation Based on a Heteronuclear Transition Metal Complex. Advanced Science, 12(19), Article 2501257. https://doi.org/10.1002/advs.202501257
- The role of ring-type structures in nonconventional luminescent polyurethane derivativesZhu, C., Meng, Y., Xu, Y., Xia, C., Jiang, N., Xu, J., & Bryce, M. R. (2025). The role of ring-type structures in nonconventional luminescent polyurethane derivatives. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 13(17), 8523-8530. https://doi.org/10.1039/d5tc00577a
- Leveraging Tumor Microenvironment to Boost Synergistic Photodynamic Therapy, Ferroptosis Anti‐Tumor Efficiency Based on a Functional Iridium(III) ComplexPei, Y., Pan, Y., Zhang, Z., Zhu, J., Sun, Y., Zhang, Q., Zhu, D., Li, G., Bryce, M. R., Wang, D., & Tang, B. Z. (2025). Leveraging Tumor Microenvironment to Boost Synergistic Photodynamic Therapy, Ferroptosis Anti‐Tumor Efficiency Based on a Functional Iridium(III) Complex. Advanced Science, 12(14), Article 2413879. https://doi.org/10.1002/advs.202413879
- Electronically Perturbed Vibrational Excitations of the Luminescing Stable Blatter RadicalBar-David, J., Daaoub, A., Chen, S., Sibug-Torres, S. M., Rocchetti, S., Kang, G., Davidson, R. J., Salthouse, R. J., Guo, C., Mueller, N. S., Sangtarash, S., Bryce, M. R., Sadeghi, H., Baumberg, J. J., Davidson, R. J., & Baumberg, J. J. (2025). Electronically Perturbed Vibrational Excitations of the Luminescing Stable Blatter Radical. ACS Nano, 19(8), 7650-7660. https://doi.org/10.1021/acsnano.4c09661
- Diiridium(III) Complexes with Fluorenylpyridyl Cyclometalating and μ 2 ‐Oxamidato Bridging Ligands and their High Efficiency Phosphorescent Solution‐Processed OLEDsM’hamedi, A., Fox, M. A., Batsanov, A. S., Al‐Attar, H. A., & Bryce, M. R. (2025). Diiridium(III) Complexes with Fluorenylpyridyl Cyclometalating and μ 2 ‐Oxamidato Bridging Ligands and their High Efficiency Phosphorescent Solution‐Processed OLEDs. European Journal of Inorganic Chemistry, 28(6), Article e202400745. https://doi.org/10.1002/ejic.202400745
- Non-Traditional Luminescent Polyurethanes of n –π Electron Hybrid Structures with Varying Separation of Aromatic RingsWang, Z., Li, Y., Zhang, H., Jiang, N., Xu, J., Zhu, D., & Bryce, M. R. (2025). Non-Traditional Luminescent Polyurethanes of n –π Electron Hybrid Structures with Varying Separation of Aromatic Rings. ACS Applied Polymer Materials, 7(18), 12337-12344. https://doi.org/10.1021/acsapm.5c02091
- Nonconventional Full-Color Luminescent Polyurethanes: Luminescence Mechanism at the Molecular Orbital LevelJiang, N., Meng, Y., Pu, X., Zhu, C., Tan, S., Xu, Y., Zhu, Y., Xu, J., & Bryce, M. R. (2025). Nonconventional Full-Color Luminescent Polyurethanes: Luminescence Mechanism at the Molecular Orbital Level. ACS Materials Letters, 7(1), 24-31. https://doi.org/10.1021/acsmaterialslett.4c02100
- Disulfide-Bridged Cationic Dinuclear Ir(III) Complex with Aggregation-Induced Emission and Glutathione-Consumption Properties for Elevating Photodynamic TherapyHuang, M., Cui, J., Wu, Q., Liu, S., Zhu, D., Li, G., Bryce, M. R., Wang, D., & Tang, B. Z. (2024). Disulfide-Bridged Cationic Dinuclear Ir(III) Complex with Aggregation-Induced Emission and Glutathione-Consumption Properties for Elevating Photodynamic Therapy. Inorganic Chemistry, 63(50), 24030-24040. https://doi.org/10.1021/acs.inorgchem.4c04571
- One-pot preparation of nonconventional luminescent polymer gels driven by polymerizationJiang, N., Pu, X., Li, K., Zhu, C., Sun, Y., Xu, Y., Zhu, Y., & Bryce, M. R. (2024). One-pot preparation of nonconventional luminescent polymer gels driven by polymerization. Polymer Chemistry, 15(40), 4101-4106. https://doi.org/10.1039/d4py00832d
- Nonconjugated Polyurethane Derivatives with Aggregation-Induced Luminochromism for Multicolor and White Photoluminescent FilmsJiang, N., Meng, Y., Zhu, C., Li, K., Li, X., Xu, Y., Xu, J., & Bryce, M. R. (2024). Nonconjugated Polyurethane Derivatives with Aggregation-Induced Luminochromism for Multicolor and White Photoluminescent Films. ACS Macro Letters, 13(10), 1226-1232. https://doi.org/10.1021/acsmacrolett.4c00534
- Long-wavelength triggered iridium( iii ) complex nanoparticles for photodynamic therapy against hypoxic cancerLiu, S., Wang, Z., Wu, Z., Chen, H., Zhu, D., Li, G., Yan, M., Bryce, M. R., & Chang, Y. (2024). Long-wavelength triggered iridium( iii ) complex nanoparticles for photodynamic therapy against hypoxic cancer. Chemical Communications, 60(73), 9938-9941. https://doi.org/10.1039/d4cc03501a
- Self-Chemiluminescence-Triggered Ir(III) Complex Photosensitizer for Photodynamic Therapy against Hypoxic TumorLiu, S., Chen, H., Wu, Q., Sun, Y., Pei, Y., Wang, Z., Zhu, D., Li, G., Bryce, M. R., & Chang, Y. (2024). Self-Chemiluminescence-Triggered Ir(III) Complex Photosensitizer for Photodynamic Therapy against Hypoxic Tumor. Inorganic Chemistry, 63(35), 16404-16417. https://doi.org/10.1021/acs.inorgchem.4c02399
- The Conductance and Thermopower Behavior of Pendent Trans -Coordinated Palladium(II) Complexes in Single-Molecule JunctionsBastante, P., Davidson, R. J., Al Malki, W., Salthouse, R. J., Cea, P., Martin, S., Batsanov, A. S., Lambert, C. J., Bryce, M. R., & Agrait, N. (2024). The Conductance and Thermopower Behavior of Pendent Trans -Coordinated Palladium(II) Complexes in Single-Molecule Junctions. ACS Omega, 9(36), 38303-38312. https://doi.org/10.1021/acsomega.4c06475
- Exciplex, Not Heavy-Atom Effect, Controls the Triplet Dynamics of a Series of Sulfur-Containing Thermally Activated Delayed Fluorescence MoleculesÖner, S., Kuila, S., Stavrou, K., Danos, A., Fox, M. A., Monkman, A. P., & Bryce, M. R. (2024). Exciplex, Not Heavy-Atom Effect, Controls the Triplet Dynamics of a Series of Sulfur-Containing Thermally Activated Delayed Fluorescence Molecules. Chemistry of Materials, 36(15), 7135-7150. https://doi.org/10.1021/acs.chemmater.4c00850
- “Three birds with one stone” nanoplatform: Efficient near‐infrared‐triggered type‑I AIE photosensitizer for mitochondria‐targeted photodynamic therapy against hypoxic tumorsLiu, S., Pei, Y., Sun, Y., Wang, Z., Chen, H., Zhu, D., Bryce, M. R., Tang, B. Z., & Chang, Y. (2024). “Three birds with one stone” nanoplatform: Efficient near‐infrared‐triggered type‑I AIE photosensitizer for mitochondria‐targeted photodynamic therapy against hypoxic tumors. Aggregate, 5(4), Article e547. https://doi.org/10.1002/agt2.547
- Recent Progress in Nonconventional Luminescent Macromolecules and their ApplicationsJiang, N., Zhu, C., Li, K., Xu, Y., & Bryce, M. R. (2024). Recent Progress in Nonconventional Luminescent Macromolecules and their Applications. Macromolecules, 57(12), 5561-5577. https://doi.org/10.1021/acs.macromol.4c00186
- Exploring the Impact of the HOMO–LUMO Gap on Molecular Thermoelectric Properties: A Comparative Study of Conjugated Aromatic, Quinoidal, and Donor–Acceptor Core SystemsBlankevoort, N., Bastante, P., Davidson, R. J., Salthouse, R. J., Daaoub, A. H. S., Cea, P., Solans, S. M., Batsanov, A. S., Sangtarash, S., Bryce, M. R., Agrait, N., & Sadeghi, H. (2024). Exploring the Impact of the HOMO–LUMO Gap on Molecular Thermoelectric Properties: A Comparative Study of Conjugated Aromatic, Quinoidal, and Donor–Acceptor Core Systems. ACS Omega, 9(7), 8471-8477. https://doi.org/10.1021/acsomega.3c09760
- Cluster-induced aggregation in polyurethane derivatives with multicolour emission and ultra-long organic room temperature phosphorescenceJiang, N., Li, K., Wang, J., Li, C., Xu, X., Xu, Y., & Bryce, M. R. (2024). Cluster-induced aggregation in polyurethane derivatives with multicolour emission and ultra-long organic room temperature phosphorescence. Journal of Materials Chemistry C, 12(3), 1040-1046. https://doi.org/10.1039/d3tc04141g
- Amphiphilic Polyurethane with Cluster-Induced Emission for Multichannel Bioimaging in Living Cell Systems.Jiang, N., Li, K., Wang, J., Zhu, Y., Zhu, C., Xu, Y., & Bryce, M. R. (2024). Amphiphilic Polyurethane with Cluster-Induced Emission for Multichannel Bioimaging in Living Cell Systems. ACS Macro Letters, 13(1), 52-57. https://doi.org/10.1021/acsmacrolett.3c00657
- Rigid and planar π-conjugated molecules leading to long-lived intramolecular charge-transfer states exhibiting thermally activated delayed fluorescenceKuila, S., Miranda-Salinas, H., Eng, J., Li, C., Bryce, M. R., Penfold, T. J., & Monkman, A. P. (2024). Rigid and planar π-conjugated molecules leading to long-lived intramolecular charge-transfer states exhibiting thermally activated delayed fluorescence. Nature Communications, 15, Article 9611. https://doi.org/10.1038/s41467-024-53740-1
- Structural Diversity in Cyclometalated Diiridium(III) Complexes with Bridging syn and anti μ2‐Oxamidato and μ2‐Dithioxamidato LigandsM’hamedi, A., Batsanov, A. S., Fox, M. A., Aguilar, J. A., & Bryce, M. R. (2023). Structural Diversity in Cyclometalated Diiridium(III) Complexes with Bridging syn and anti μ2‐Oxamidato and μ2‐Dithioxamidato Ligands. European Journal of Inorganic Chemistry, 26(34), Article e202300423. https://doi.org/10.1002/ejic.202300423
- Multicolor Luminescence of a Polyurethane Derivative Driven by Heat/Light-Induced AggregationJiang, N., Li, K., Xie, W., Zhang, S., Li, X., Hu, Y., Xu, Y., Liu, X., & Bryce, M. R. (2023). Multicolor Luminescence of a Polyurethane Derivative Driven by Heat/Light-Induced Aggregation. Macromolecules, 56(19), 7721-7728. https://doi.org/10.1021/acs.macromol.3c01345
- Near-Infrared Afterglow ONOO–-Triggered Nanoparticles for Real-Time Monitoring and Treatment of Early Ischemic StrokeZhang, L., Wang, Y., Liao, Y., Zhang, Q., Liu, X., Zhu, D., Feng, H., Bryce, M. R., & Ren, L. (2023). Near-Infrared Afterglow ONOO–-Triggered Nanoparticles for Real-Time Monitoring and Treatment of Early Ischemic Stroke. ACS Applied Materials & Interfaces, 15(39). https://doi.org/10.1021/acsami.3c08033
- Aggregation-Induced Emission (AIE), Life and HealthWang, H., Li, Q., Alam, P., Bai, H., Bhalla, V., Bryce, M. R., Cao, M., Chen, C., Chen, S., Chen, X., Chen, Y., Chen, Z., Dang, D., Ding, D., Ding, S., Duo, Y., Gao, M., He, W., He, X., … Tang, B. Z. (2023). Aggregation-Induced Emission (AIE), Life and Health. ACS Nano, 17(15), 14347–14405. https://doi.org/10.1021/acsnano.3c03925
- Electronic Conductance and Thermopower of Cross-Conjugated and Skipped-Conjugated Molecules in Single-Molecule JunctionsSalthouse, R. J., Hurtado-Gallego, J., Grace, I. M., Davidson, R., Alshammari, O., Agraït, N., Lambert, C. J., & Bryce, M. R. (2023). Electronic Conductance and Thermopower of Cross-Conjugated and Skipped-Conjugated Molecules in Single-Molecule Junctions. The Journal of Physical Chemistry C, 127(28), 13751-13758. https://doi.org/10.1021/acs.jpcc.3c00742
- A review of fused-ring carbazole derivatives as emitter and/or host materials in organic light emitting diode (OLED) applicationsOner, S., & Bryce, M. R. (2023). A review of fused-ring carbazole derivatives as emitter and/or host materials in organic light emitting diode (OLED) applications. Materials Chemistry Frontiers, 7(19), 4304–4338. https://doi.org/10.1039/d3qm00399j
- Oxidation State Tuning of Room Temperature Phosphorescence and Delayed Fluorescence in Phenothiazine and Phenothiazine‐5,5‐dioxide DimersWright, I. A., Etherington, M. K., Batsanov, A. S., Monkman, A. P., & Bryce, M. R. (2023). Oxidation State Tuning of Room Temperature Phosphorescence and Delayed Fluorescence in Phenothiazine and Phenothiazine‐5,5‐dioxide Dimers. Chemistry – A European Journal, 29(30), Article e202300428. https://doi.org/10.1002/chem.202300428
- Planar aromatic anchors control the electrical conductance of gold|molecule|graphene junctionsO’Driscoll, L. J., Jay, M., Robinson, B. J., Sadeghi, H., Wang, X., Penhale-Jones, B., Bryce, M. R., & Lambert, C. J. (2023). Planar aromatic anchors control the electrical conductance of gold|molecule|graphene junctions. Nanoscale Advances, 5(8), 2299-2306. https://doi.org/10.1039/d2na00873d
- Fine‐Tuning the Photophysics of Donor‐Acceptor (D‐A 3 ) Thermally Activated Delayed Fluorescence Emitters Using IsomerisationL. dos Santos, P., de Sa Pereira, D., Eng, J., Ward, J. S., Bryce, M. R., Penfold, T. J., & Monkman, A. P. (2023). Fine‐Tuning the Photophysics of Donor‐Acceptor (D‐A 3 ) Thermally Activated Delayed Fluorescence Emitters Using Isomerisation. ChemPhotoChem, 7(2), Article e202200248. https://doi.org/10.1002/cptc.202200248
- Self-assembled nanoparticles based on cationic mono-/AIE tetra-nuclear Ir(III) complexes: long wavelength absorption/near-infrared emission photosensitizers for photodynamic therapyWang, Z., Li, L., Wang, W., Wang, R., Li, G., Bian, H., Zhu, D., & Bryce, M. R. (2023). Self-assembled nanoparticles based on cationic mono-/AIE tetra-nuclear Ir(III) complexes: long wavelength absorption/near-infrared emission photosensitizers for photodynamic therapy. Dalton Transactions, 52(6), 1595-1601. https://doi.org/10.1039/d2dt03809a
- Full thermoelectric characterization of a single moleculeGemma, A., Tabatabaei, F., Drechsler, U., Zulji, A., Dekkiche, H., Mosso, N., Niehaus, T., Bryce, M. R., Merabia, S., & Gotsmann, B. (2023). Full thermoelectric characterization of a single molecule. Nature Communications, 14(1), Article 3868. https://doi.org/10.1038/s41467-023-39368-7
- Quantum interference dependence on molecular configurations for cross-conjugated systems in single-molecule junctionsHurtado-Gallego, J., Davidson, R., Grace, I. M., Rincón-García, L., Batsanov, A. S., Bryce, M. R., Lambert, C. J., & Agraït, N. (2022). Quantum interference dependence on molecular configurations for cross-conjugated systems in single-molecule junctions. Molecular Systems Design & Engineering, 7(10), 1287-1293. https://doi.org/10.1039/d2me00074a
- Electrostatic Fermi level tuning in large-scale self-assembled monolayers of oligo(phenylene–ethynylene) derivativesWang, X., Ismael, A., Ning, S., Althobaiti, H., Al-Jobory, A., Girovsky, J., Astier, H. P., O’Driscoll, L. J., Bryce, M. R., Lambert, C. J., & Ford, C. J. (2022). Electrostatic Fermi level tuning in large-scale self-assembled monolayers of oligo(phenylene–ethynylene) derivatives. Nanoscale Horizons, 7(10), 1201-1209. https://doi.org/10.1039/d2nh00241h
- Intramolecular Hydrogen Bonding in Thermally Activated Delayed Fluorescence Emitters: Is There Evidence Beyond Reasonable Doubt?Hempe, M., Kukhta, N. A., Danos, A., Batsanov, A. S., Monkman, A. P., & Bryce, M. R. (2022). Intramolecular Hydrogen Bonding in Thermally Activated Delayed Fluorescence Emitters: Is There Evidence Beyond Reasonable Doubt? The Journal of Physical Chemistry Letters, 13(35), 8221-8227. https://doi.org/10.1021/acs.jpclett.2c00907
- Asymmetrical‐Dendronized TADF Emitters for Efficient Non‐doped Solution‐Processed OLEDs by Eliminating Degenerate Excited States and Creating Solely Thermal Equilibrium RoutesLi, C., Harrison, A. K., Liu, Y., Zhao, Z., Zeng, C., Dias, F. B., Ren, Z., Yan, S., & Bryce, M. R. (2022). Asymmetrical‐Dendronized TADF Emitters for Efficient Non‐doped Solution‐Processed OLEDs by Eliminating Degenerate Excited States and Creating Solely Thermal Equilibrium Routes. Angewandte Chemie International Edition, 61(19), Article e202115140. https://doi.org/10.1002/anie.202115140
- TADF dendronized polymer with vibrationally enhanced direct spin-flip between charge-transfer states for efficient non-doped solution-processed OLEDsLi, C., Harrison, A. K., Liu, Y., Zhao, Z., Dias, F. B., Zeng, C., Yan, S., Bryce, M. R., & Ren, Z. (2022). TADF dendronized polymer with vibrationally enhanced direct spin-flip between charge-transfer states for efficient non-doped solution-processed OLEDs. Chemical Engineering Journal, 435, Article 134924. https://doi.org/10.1016/j.cej.2022.134924
- AIE-active Ir(iii) complexes functionalised with a cationic Schiff base ligand: synthesis, photophysical properties and applications in photodynamic therapyLiu, S., Han, J., Wang, W., Chang, Y., Wang, R., Wang, Z., Li, G., Zhu, D., & Bryce, M. R. (2022). AIE-active Ir(iii) complexes functionalised with a cationic Schiff base ligand: synthesis, photophysical properties and applications in photodynamic therapy. Dalton Transactions, 51(42), 16119-16125. https://doi.org/10.1039/d2dt02960j
- Thermoelectric Enhancement in Single Organic Radical MoleculesHurtado-Gallego, J., Sangtarash, S., Davidson, R., Rincón-García, L., Daaoub, A., Rubio-Bollinger, G., Lambert, C. J., Oganesyan, V. S., Bryce, M. R., Agraït, N., & Sadeghi, H. (2022). Thermoelectric Enhancement in Single Organic Radical Molecules. Nano Letters, 22(3). https://doi.org/10.1021/acs.nanolett.1c03698
- AIE-active iridium(iii) complex integrated with upconversion nanoparticles for NIR-irradiated photodynamic therapyLiu, S., Han, J., Chang, Y., Wang, W., Wang, R., Wang, Z., Li, G., Zhu, D., & Bryce, M. R. (2022). AIE-active iridium(iii) complex integrated with upconversion nanoparticles for NIR-irradiated photodynamic therapy. Chemical Communications, 58(72), 10056-10059. https://doi.org/10.1039/d2cc03622c
- Thermoelectric properties of organic thin films enhanced by π–π stackingWang, X., Sangtarash, S., Lamantia, A., Dekkiche, H., Forcieri, L., Kolosov, O. V., Jarvis, S. P., Bryce, M. R., Lambert, C. J., Sadeghi, H., & Robinson, B. J. (2022). Thermoelectric properties of organic thin films enhanced by π–π stacking. Journal of Physics: Energy, 4(2). https://doi.org/10.1088/2515-7655/ac55a3
- A review of functional linear carbon chains (oligoynes, polyynes, cumulenes) and their applications as molecular wires in molecular electronics and optoelectronicsBryce, M. R. (2021). A review of functional linear carbon chains (oligoynes, polyynes, cumulenes) and their applications as molecular wires in molecular electronics and optoelectronics. Journal of Materials Chemistry C, 9(33), 10524-10546. https://doi.org/10.1039/d1tc01406d
- Allocation of Ambipolar Charges on an Organic Diradical with a Vinylene–Phenylenediyne BridgeMayorga-Burrezo, P., Bejarano, F., Calbo, J., Zhao, X., De Sousa, J. A., Lloveras, V., Bryce, M. R., Ortí, E., Veciana, J., Rovira, C., & Crivillers, N. (2021). Allocation of Ambipolar Charges on an Organic Diradical with a Vinylene–Phenylenediyne Bridge. Journal of Physical Chemistry Letters, 12(26), 6159-6164. https://doi.org/10.1021/acs.jpclett.1c01565
- Vibrational Damping Reveals Vibronic Coupling in Thermally Activated Delayed Fluorescence MaterialsHempe, M., Kukhta, N. A., Danos, A., Fox, M. A., Batsanov, A. S., Monkman, A. P., & Bryce, M. R. (2021). Vibrational Damping Reveals Vibronic Coupling in Thermally Activated Delayed Fluorescence Materials. Chemistry of Materials, 33(9), 3066-3080. https://doi.org/10.1021/acs.chemmater.0c03783
- Rational design of iridium–porphyrin conjugates for novel synergistic photodynamic and photothermal therapy anticancer agentsZhang, L., Geng, Y., Li, L., Tong, X., Liu, S., Liu, X., Su, Z., Xie, Z., Zhu, D., & Bryce, M. R. (2021). Rational design of iridium–porphyrin conjugates for novel synergistic photodynamic and photothermal therapy anticancer agents. Chemical Science, 12(16), 5918-5925. https://doi.org/10.1039/d1sc00126d
- Conformational Dependence of Triplet Energies in Rotationally Hindered N‐ and S‐Heterocyclic Dimers: New Design and Measurement Rules for High Triplet Energy OLED Host MaterialsWright, I. A., Danos, A., Montanaro, S., Batsanov, A. S., Monkman, A. P., & Bryce, M. R. (2021). Conformational Dependence of Triplet Energies in Rotationally Hindered N‐ and S‐Heterocyclic Dimers: New Design and Measurement Rules for High Triplet Energy OLED Host Materials. Chemistry - A European Journal, 27(21), 6545-6556. https://doi.org/10.1002/chem.202100036
- Extended curly arrow rules to rationalise and predict structural effects on quantum interference in molecular junctionsO’Driscoll, L. J., & Bryce, M. R. (2021). Extended curly arrow rules to rationalise and predict structural effects on quantum interference in molecular junctions. Nanoscale, 13(2), 1103-1123. https://doi.org/10.1039/d0nr07819k
- Dual emission in purely organic materials for optoelectronic applicationsKukhta, N. A., & Bryce, M. R. (2021). Dual emission in purely organic materials for optoelectronic applications. Materials Horizons., 8(1), 33-55. https://doi.org/10.1039/d0mh01316a
- Heteroatom Effects on Quantum Interference in Molecular Junctions: Modulating Antiresonances by Molecular DesignO’Driscoll, L. J., Sangtarash, S., Xu, W., Daaoub, A., Hong, W., Sadeghi, H., & Bryce, M. R. (2021). Heteroatom Effects on Quantum Interference in Molecular Junctions: Modulating Antiresonances by Molecular Design. The Journal of Physical Chemistry C, 125(31), 17385-17391. https://doi.org/10.1021/acs.jpcc.1c04242
- Cyclophane Molecules Exhibiting Thermally Activated Delayed Fluorescence: Linking Donor Units to Influence Molecular ConformationHempe, M., Harrison, A. K., Ward, J. S., Batsanov, A. S., Fox, M. A., Dias, F. B., & Bryce, M. R. (2021). Cyclophane Molecules Exhibiting Thermally Activated Delayed Fluorescence: Linking Donor Units to Influence Molecular Conformation. Journal of Organic Chemistry, 86(1), 429-445. https://doi.org/10.1021/acs.joc.0c02174
- A review of oligo(arylene ethynylene) derivatives in molecular junctionsO’Driscoll, L. J., & Bryce, M. R. (2021). A review of oligo(arylene ethynylene) derivatives in molecular junctions. Nanoscale, 13(24), 10668-10711. https://doi.org/10.1039/d1nr02023d
- Supramolecular oligourethane gels as light-harvesting antennae: achieving multicolour luminescence and white-light emission through FRETJiang, N., Wang, R., You, X., Geng, Y., Zhu, D., Zhang, N., & Bryce, M. R. (2021). Supramolecular oligourethane gels as light-harvesting antennae: achieving multicolour luminescence and white-light emission through FRET. Journal of Materials Chemistry C, 9(38). https://doi.org/10.1039/d1tc03105h
- Resonance-enhanced charge delocalization in carbazole-oligoyne-oxadiazole conjugatesZieleniewska, A., Zhao, X., Bauroth, S., Wang, C., Batsanov, A., Calderson, C., Kahnt, A., Clark, T., Bryce, M., & Guldi, D. (2020). Resonance-enhanced charge delocalization in carbazole-oligoyne-oxadiazole conjugates. Journal of the American Chemical Society, 142(44), 18769-18781. https://doi.org/10.1021/jacs.0c04003
- Supramolecular oligourethane gel as a highly selective fluorescent “on–off–on” sensor for ionsFeng, Y., Jiang, N., Zhu, D., Su, Z., & Bryce, M. R. (2020). Supramolecular oligourethane gel as a highly selective fluorescent “on–off–on” sensor for ions. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 8(33), 11540-11545. https://doi.org/10.1039/d0tc02381g
- Electronic conductance and thermopower of single-molecule junctions of oligo(phenyleneethynylene) derivativesDekkiche, H., Gemma, A., Tabatabaei, F., Batsanov, A. S., Niehaus, T., Gotsmann, B., & Bryce, M. R. (2020). Electronic conductance and thermopower of single-molecule junctions of oligo(phenyleneethynylene) derivatives. Nanoscale, 12(36), 18908-18917. https://doi.org/10.1039/d0nr04413j
- Supramolecular Oligourethane Gel with Multicolor Luminescence Controlled by Mechanically Sensitive Hydrogen-BondingJiang, N., Ruan, S., Liu, X., Zhu, D., Li, B., & Bryce, M. R. (2020). Supramolecular Oligourethane Gel with Multicolor Luminescence Controlled by Mechanically Sensitive Hydrogen-Bonding. Chemistry of Materials, 32(13), 5776-5784. https://doi.org/10.1021/acs.chemmater.0c01620
- Exploring the thermoelectric properties of oligo(phenylene-ethynylene) derivativesChen, H., Sangtarash, S., Li, G., Gantenbein, M., Cao, W., Alqorashi, A., Liu, J., Zhang, C., Zhang, Y., Chen, L., Chen, Y., Olsen, G., Sadeghi, H., Bryce, M. R., Lambert, C. J., & Hong, W. (2020). Exploring the thermoelectric properties of oligo(phenylene-ethynylene) derivatives. Nanoscale, 12(28), 15150-15156. https://doi.org/10.1039/d0nr03303k
- Connectivity dependent thermopower of bridged biphenyl molecules in single-molecule junctionsGrace, I. M., Olsen, G., Hurtado-Gallego, J., Rincón-García, L., Rubio-Bollinger, G., Bryce, M. R., Agraït, N., & Lambert, C. J. (2020). Connectivity dependent thermopower of bridged biphenyl molecules in single-molecule junctions. Nanoscale, 12(27), 14682-14688. https://doi.org/10.1039/d0nr04001k
- Blue-emitting thermoreversible oligourethane gelators with aggregation-induced emission propertiesJiang, N., Zhu, D., Su, Z., & Bryce, M. R. (2020). Blue-emitting thermoreversible oligourethane gelators with aggregation-induced emission properties. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 8(15), 5137-5142. https://doi.org/10.1039/d0tc00757a
- Bright red aggregation-induced emission nanoparticles for multifunctional applications in cancer therapyZhang, L., Che, W., Yang, Z., Liu, X., Liu, S., Xie, Z., Zhu, D., Su, Z., Tang, B. Z., & Bryce, M. R. (2020). Bright red aggregation-induced emission nanoparticles for multifunctional applications in cancer therapy. Chemical Science, 11(9), 2369-2374. https://doi.org/10.1039/c9sc06310b
- Unusual dual-emissive heteroleptic iridium complexes incorporating TADF cyclometalating ligandsBenjamin, H., Zheng, Y., Kozhevnikov, V. N., Siddle, J. S., O’Driscoll, L. J., Fox, M. A., Batsanov, A. S., Griffiths, G. C., Dias, F. B., Monkman, A. P., & Bryce, M. R. (2020). Unusual dual-emissive heteroleptic iridium complexes incorporating TADF cyclometalating ligands. Dalton Transactions, 49(7), 2190-2208. https://doi.org/10.1039/c9dt04672k
- Dinuclear metal complexes: multifunctional properties and applicationsLi, G., Zhu, D., Wang, X., Su, Z., & Bryce, M. R. (2020). Dinuclear metal complexes: multifunctional properties and applications. Chemical Society Reviews, 49(3), 765-838. https://doi.org/10.1039/c8cs00660a
- Carbazole‐Based Tetrapodal Anchor Groups for Gold Surfaces: Synthesis and Conductance PropertiesBryce, M. R., O’Driscoll, L., Wang, X., Jay, M., Batsanov, A., Sadeghi, H., Lambert, C., & Robinson, B. (2020). Carbazole‐Based Tetrapodal Anchor Groups for Gold Surfaces: Synthesis and Conductance Properties. Angewandte Chemie International Edition, 59(2), 882-889. https://doi.org/10.1002/anie.201911652
- Exploring antiaromaticity in single-molecule junctions formed from biphenylene derivativesGantenbein, M., Li, X., Sangtarash, S., Bai, J., Olsen, G., Alqorashi, A., Hong, W., Lambert, C. J., & Bryce, M. R. (2019). Exploring antiaromaticity in single-molecule junctions formed from biphenylene derivatives. Nanoscale, 11(43), 20659-20666. https://doi.org/10.1039/c9nr05375a
- Achieving Conformational Control in RTP and TADF Emitters by Functionalization of the Central CoreKukhta, N., Huang, R., Batsanov, A., Bryce, M., & Dias, F. (2019). Achieving Conformational Control in RTP and TADF Emitters by Functionalization of the Central Core. Journal of Physical Chemistry C, 123(43), 26536-26546. https://doi.org/10.1021/acs.jpcc.9b08238
- Balancing charge-transfer strength and triplet states for deep-blue thermally activated delayed fluorescence with an unconventional electron rich dibenzothiophene acceptorHuang, R., Kukhta, N., Ward, J., Danos, A., Batsanov, A., Bryce, M., & Dias, F. (2019). Balancing charge-transfer strength and triplet states for deep-blue thermally activated delayed fluorescence with an unconventional electron rich dibenzothiophene acceptor. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 7(42), 13224-13234. https://doi.org/10.1039/c9tc02175b
- Strategic modification of ligands for remarkable piezochromic luminescence (PCL) based on a neutral Ir(iii) phosphorLi, D., Li, G., Xie, J., Zhu, D., Su, Z., & Bryce, M. R. (2019). Strategic modification of ligands for remarkable piezochromic luminescence (PCL) based on a neutral Ir(iii) phosphor. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 7(35), 10876-10880. https://doi.org/10.1039/c9tc03646f
- Molecular Design Strategies for Color Tuning of Blue TADF EmittersStachelek, P., Ward, J. S., dos Santos, P. L., Danos, A., Colella, M., Haase, N., Raynes, S. J., Batsanov, A. S., Bryce, M. R., Monkman, A. P., & Brook, P. (2019). Molecular Design Strategies for Color Tuning of Blue TADF Emitters. ACS Applied Materials and Interfaces, 11(30), 27125-27133. https://doi.org/10.1021/acsami.9b06364
- Delayed Blue Fluorescence via Upper-triplet State Crossing from C-C Bonded Donor-Acceptor Charge-Transfer Molecules with Azatriangulene coresWard, J., Kukhta, N., Dos Santos, P., Congrave, D., Batsanov, A., Monkman, A., & Bryce, M. (2019). Delayed Blue Fluorescence via Upper-triplet State Crossing from C-C Bonded Donor-Acceptor Charge-Transfer Molecules with Azatriangulene cores. Chemistry of Materials, 31(17), 6684-6695. https://doi.org/10.1021/acs.chemmater.9b01184
- Persistent Dimer Emission in Thermally Activated Delayed Fluorescence MaterialsEtherington, M. K., Kukhta, N. A., Higginbotham, H. F., Danos, A., Bismillah, A. N., Graves, D. R., McGonigal, P. R., Haase, N., Morherr, A., Batsanov, A. S., Pflumm, C., Bhalla, V., Bryce, M. R., & Monkman, A. P. (2019). Persistent Dimer Emission in Thermally Activated Delayed Fluorescence Materials. Journal of Physical Chemistry C, 123(17), 11109-11117. https://doi.org/10.1021/acs.jpcc.9b01458
- AIE Multinuclear Ir(III) Complexes for Biocompatible Organic Nanoparticles with Highly Enhanced Photodynamic PerformanceZhang, L., Li, Y., Che, W., Zhu, D., Li, G., Xie, Z., Song, N., Liu, S., Tang, B. Z., Liu, X., Su, Z., & Bryce, M. R. (2019). AIE Multinuclear Ir(III) Complexes for Biocompatible Organic Nanoparticles with Highly Enhanced Photodynamic Performance. Advanced Science, 6(5), Article 1802050. https://doi.org/10.1002/advs.201802050
- Transition from Tunneling Leakage Current to Molecular Tunneling in Single-Molecule JunctionsLiu, J., Zhao, X., Zheng, J., Huang, X., Tang, Y., Wang, F., Li, R., Pi, J., Huang, C., Wang, L., Yang, Y., Shi, J., Mao, B., Tian, Z., Bryce, M. R., & Hong, W. (2019). Transition from Tunneling Leakage Current to Molecular Tunneling in Single-Molecule Junctions. Chem, 5(2), 390-401. https://doi.org/10.1016/j.chempr.2018.11.002
- Reversible tricolour luminescence switching based on a piezochromic iridium(iii) complexYang, T., Wang, Y., Liu, X., Li, G., Che, W., Zhu, D., Su, Z., & Bryce, M. R. (2019). Reversible tricolour luminescence switching based on a piezochromic iridium(iii) complex. Chemical Communications, 55(97), 14582-14585. https://doi.org/10.1039/c9cc08545a
- Highly luminescent 2-phenylpyridine-free diiridium complexes with bulky 1,2-diarylimidazole cyclometalating ligandsCongrave, D. G., Batsanov, A. S., & Bryce, M. R. (2018). Highly luminescent 2-phenylpyridine-free diiridium complexes with bulky 1,2-diarylimidazole cyclometalating ligands. Dalton Transactions, 47(46), 16524-16533. https://doi.org/10.1039/c8dt04043e
- Intramolecular Charge Transfer Controls Switching Between Room Temperature Phosphorescence and Thermally Activated Delayed FluorescenceChen, C., Huang, R., Batsanov, A., Pander, P., Hsu, Y., Chi, Z., Dias, F., & Bryce, M. R. (2018). Intramolecular Charge Transfer Controls Switching Between Room Temperature Phosphorescence and Thermally Activated Delayed Fluorescence. Angewandte Chemie, 130(50), 16645-16649. https://doi.org/10.1002/ange.201809945
- Importance of Chromophore Rigidity on the Efficiency of Blue Thermally Activated Delayed Fluorescence EmittersKukhta, N. A., Batsanov, A. S., Bryce, M. R., & Monkman, A. P. (2018). Importance of Chromophore Rigidity on the Efficiency of Blue Thermally Activated Delayed Fluorescence Emitters. Journal of Physical Chemistry C, 122(50), 28564-28575. https://doi.org/10.1021/acs.jpcc.8b10867
- Thermoelectric Properties of 2,7-Dipyridylfluorene Derivatives in Single-Molecule JunctionsYzambart, G., Rincón-García, L., Al-Jobory, A. A., Ismael, A. K., Rubio-Bollinger, G., Lambert, C. J., Agraït, N., & Bryce, M. R. (2018). Thermoelectric Properties of 2,7-Dipyridylfluorene Derivatives in Single-Molecule Junctions. Journal of Physical Chemistry C, 122(48), 27198-27204. https://doi.org/10.1021/acs.jpcc.8b08488
- New Mixed-C^N Ligand Tris-cyclometalated Ir(III) Complexes for Highly-Efficient Green Organic Light-Emitting Diodes with Low Efficiency Roll-offMa, X., Liang, J., Bai, F., Ye, K., Xu, J., Zhu, D., & Bryce, M. R. (2018). New Mixed-C^N Ligand Tris-cyclometalated Ir(III) Complexes for Highly-Efficient Green Organic Light-Emitting Diodes with Low Efficiency Roll-off. European Journal of Inorganic Chemistry, 2018(42), 4614-4621. https://doi.org/10.1002/ejic.201800550
- Bond Rotations and Heteroatom Effects in Donor-Acceptor-Donor Molecules: Implications for Thermally Activated Delayed Fluorescence and Room Temperature PhosphorescenceWard, J. S., Nobuyasu, R. S., Fox, M. A., Batsanov, A. S., Santos, J., Dias, F. B., & Bryce, M. R. (2018). Bond Rotations and Heteroatom Effects in Donor-Acceptor-Donor Molecules: Implications for Thermally Activated Delayed Fluorescence and Room Temperature Phosphorescence. Journal of Organic Chemistry, 83(23), 14431-14442. https://doi.org/10.1021/acs.joc.8b02187
- Intramolecular Pi-Pi Interactions with a Chiral Auxiliary Ligand Control Diastereoselectivity in a Cyclometalated Ir(III) ComplexCongrave, D. G., Batsanov, A. S., Du, M., Liu, Y., Zhu, D., & Bryce, M. R. (2018). Intramolecular Pi-Pi Interactions with a Chiral Auxiliary Ligand Control Diastereoselectivity in a Cyclometalated Ir(III) Complex. Inorganic Chemistry, 57(20), 12836-12849. https://doi.org/10.1021/acs.inorgchem.8b02034
- Polyurethane Derivatives for Highly Sensitive and Selective Fluorescent Detection of 2,4,6-Trinitrophenol (TNP)Jiang, N., Li, G., Che, W., Zhu, D., Su, Z., & Bryce, M. R. (2018). Polyurethane Derivatives for Highly Sensitive and Selective Fluorescent Detection of 2,4,6-Trinitrophenol (TNP). Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 42(6), 11287-11291. https://doi.org/10.1039/c8tc04250k
- Synthesis of Tetracyclic 2,3-Dihydro-1,3-diazepines from a Dinitrodibenzothiophene DerivativeMontanaro, S., Wright, I. A., Batsanov, A. S., & Bryce, M. R. (2018). Synthesis of Tetracyclic 2,3-Dihydro-1,3-diazepines from a Dinitrodibenzothiophene Derivative. Journal of Organic Chemistry, 83(19), 12320-12326. https://doi.org/10.1021/acs.joc.8b02029
- The influence of molecular conformation on the photophysics of organic room temperature phosphorescent luminophoresHuang, R., Ward, J. S., Kukhta, N. A., Avó, J., Gibson, J., Penfold, T., Lima, J. C., Batsanov, A. S., Berberan-Santos, M. N., Bryce, M. R., & Dias, F. B. (2018). The influence of molecular conformation on the photophysics of organic room temperature phosphorescent luminophores. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 10(34), 9238-9247. https://doi.org/10.1039/c8tc02987c
- Heteroatom-Induced Molecular Asymmetry Tunes Quantum Interference in Charge Transport through Single-Molecule JunctionsYang, Y., Gantenbein, M., Alqorashi, A., Wei, J., Sangtarash, S., Hu, D., Sadeghi, H., Zhang, R., Pi, J., Chen, L., Huang, X., Li, R., Liu, J., Shi, J., Hong, W., Lambert, C. J., & Bryce, M. R. (2018). Heteroatom-Induced Molecular Asymmetry Tunes Quantum Interference in Charge Transport through Single-Molecule Junctions. Journal of Physical Chemistry C, 122(26), 14965-14970. https://doi.org/10.1021/acs.jpcc.8b03023
- Triazatruxene: A rigid central donor unit for a D-A3 thermally activated delayed fluorescence material exhibiting sub-microsecond reverse intersystem crossing and unity quantum yield via multiple singlet-triplet state pairsdos Santos, P. L., Ward, J. S., Congrave, D. G., Batsanov, A. S., Eng, J., Stacey, J. E., Penfold, T. J., Monkman, A. P., & Bryce, M. R. (2018). Triazatruxene: A rigid central donor unit for a D-A3 thermally activated delayed fluorescence material exhibiting sub-microsecond reverse intersystem crossing and unity quantum yield via multiple singlet-triplet state pairs. Advanced Science, 5(6), Article 1700989. https://doi.org/10.1002/advs.201700989
- Aggregation-Induced Long-Lived Phosphorescence in Non-Conjugated Polyurethane Derivatives at 77 KJiang, N., Li, G., Zhang, B., Zhu, D., Su, Z., & Bryce, M. (2018). Aggregation-Induced Long-Lived Phosphorescence in Non-Conjugated Polyurethane Derivatives at 77 K. Macromolecules, 51(11), 4178-4184. https://doi.org/10.1021/acs.macromol.8b00715
- Conformationally-Restricted Bicarbazoles with Phenylene Bridges Displaying Deep-Blue Emission and High Triplet Energies: Systematic Structure–Property RelationshipsWright, I., Al-Attar, H., Batsanov, A., Monkman, A., & Bryce, M. (2018). Conformationally-Restricted Bicarbazoles with Phenylene Bridges Displaying Deep-Blue Emission and High Triplet Energies: Systematic Structure–Property Relationships. Physical Chemistry Chemical Physics, 20(17), 11867-11875. https://doi.org/10.1039/c8cp01636d
- Selective sensing of 2,4,6-trinitrophenol (TNP) in aqueous media with “aggregation-induced emission enhancement” (AIEE)-active iridium(III) complexesChe, W., Li, G., Liu, X., Shao, K., Zhu, D., Su, Z., & Bryce, M. (2018). Selective sensing of 2,4,6-trinitrophenol (TNP) in aqueous media with “aggregation-induced emission enhancement” (AIEE)-active iridium(III) complexes. Chemical Communications, 54(14), 1730-1733. https://doi.org/10.1039/c7cc08832a
- Sky-blue emitting bridged diiridium complexes: beneficial effects of intramolecular π–π stackingCongrave, D. G., Hsu, Y., Batsanov, A. S., Beeby, A., & Bryce, M. R. (2018). Sky-blue emitting bridged diiridium complexes: beneficial effects of intramolecular π–π stacking. Dalton Transactions, 47(6), 2086-2098. https://doi.org/10.1039/c7dt04201a
- Fast Data Sorting with Modified Principal Component Analysis to Distinguish Unique Single Molecular Break Junction TrajectoriesHamill, J., Zhao, X., Mészáros, G., Bryce, M., & Arenz, M. (2018). Fast Data Sorting with Modified Principal Component Analysis to Distinguish Unique Single Molecular Break Junction Trajectories. Physical Review Letters, 120(1), Article 016601. https://doi.org/10.1103/physrevlett.120.016601
- Determination of standard redox rate constants of OLED active compounds by electrochemical impedance spectroscopyChulkin, P., Lapkowski, M., Bryce, M. R., Santos, J., & Data, P. (2017). Determination of standard redox rate constants of OLED active compounds by electrochemical impedance spectroscopy. Electrochimica Acta, 258, 1160-1172. https://doi.org/10.1016/j.electacta.2017.11.171
- An AIE-active phosphorescent Ir(III) complex with piezochromic luminescence (PCL) and its application for monitoring volatile organic compounds (VOCs)Jiang, Y., Li, G., Zhu, D., Su, Z., & Bryce, M. R. (2017). An AIE-active phosphorescent Ir(III) complex with piezochromic luminescence (PCL) and its application for monitoring volatile organic compounds (VOCs). Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 5(46), 12189-12193. https://doi.org/10.1039/c7tc04066k
- Radical Enhanced Charge Transport in Single-Molecule Phenothiazine Electrical JunctionsLiu, J., Zhao, X., Al-Galiby, Q., Huang, X., Zheng, J., Li, R., Huang, C., Yang, Y., Shi, J., Manrique, D. Z., Lambert, C. J., Bryce, M. R., & Hong, W. (2017). Radical Enhanced Charge Transport in Single-Molecule Phenothiazine Electrical Junctions. Angewandte Chemie International Edition, 56(42), 13061-13065. https://doi.org/10.1002/anie.201707710
- Thermally Activated Delayed Fluorescence in Cu(I) Complexes Originating from Restricted Molecular VibrationsLi, G., Nobuyasu, R., Zhang, B., Geng, Y., Yao, B., Xie, Z., Zhu, D., Shan, G., Che, W., Yan, L., Su, Z., Dias, F., & Bryce, M. (2017). Thermally Activated Delayed Fluorescence in Cu(I) Complexes Originating from Restricted Molecular Vibrations. Chemistry - A European Journal, 23(49), 11761-11766. https://doi.org/10.1002/chem.201701862
- Pyridylpyrazole N^N Ligands Combined with Sulfonyl-Functionalised Cyclometalating Ligands for Blue-Emitting Iridium(III) Complexes and Solution-Processable PhOLEDsBenjamin, H., Fox, M. A., Batsanov, A. S., Al-Attar, H. A., Li, C., Ren, Z., Monkman, A. P., & Bryce, M. R. (2017). Pyridylpyrazole N^N Ligands Combined with Sulfonyl-Functionalised Cyclometalating Ligands for Blue-Emitting Iridium(III) Complexes and Solution-Processable PhOLEDs. Dalton Transactions, 46, 10996-11007. https://doi.org/10.1039/c7dt02289a
- Insulated molecular wires: inhibiting orthogonal contacts in metal complex based molecular junctionsAl-Owaedi, O. A., Bock, S., Milan, D. C., Oerthel, M., Inkpen, M. S., Yufit, D. S., Sobolev, A. N., Long, N. J., Albrecht, T., Higgins, S. J., Bryce, M. R., Nichols, R. J., Lambert, C. J., & Low, P. J. (2017). Insulated molecular wires: inhibiting orthogonal contacts in metal complex based molecular junctions. Nanoscale, 9(28), 9902-9912. https://doi.org/10.1039/c7nr01829k
- Photophysics of an Asymmetric Donor–Acceptor–Donor′ TADF Molecule and Reinterpretation of Aggregation-Induced TADF Emission in These MaterialsAydemir, M., Xu, S., Chen, C., Bryce, M. R., Chi, Z., & Monkman, A. P. (2017). Photophysics of an Asymmetric Donor–Acceptor–Donor′ TADF Molecule and Reinterpretation of Aggregation-Induced TADF Emission in These Materials. Journal of Physical Chemistry C, 121(33), 17764-17772. https://doi.org/10.1021/acs.jpcc.7b06299
- Optical and Polarity Control of Donor–Acceptor Conformation and Their Charge-Transfer States in Thermally Activated Delayed-Fluorescence Moleculesdos Santos, P. L., Ward, J. S., Batsanov, A. S., Bryce, M. R., & Monkman, A. P. (2017). Optical and Polarity Control of Donor–Acceptor Conformation and Their Charge-Transfer States in Thermally Activated Delayed-Fluorescence Molecules. Journal of Physical Chemistry C, 121(30), 16462-16469. https://doi.org/10.1021/acs.jpcc.7b03672
- Charge-Gating Dibenzothiophene-S,S-dioxide Bridges in Electron Donor–Bridge–Acceptor ConjugatesYzambart, G., Zieleniewska, A., Bauroth, S., Clark, T., Bryce, M. R., & Guldi, D. M. (2017). Charge-Gating Dibenzothiophene-S,S-dioxide Bridges in Electron Donor–Bridge–Acceptor Conjugates. Journal of Physical Chemistry C, 121(25), 13557–13569. https://doi.org/10.1021/acs.jpcc.7b03889
- Quantum interference and heteroaromaticity of para- and meta-linked bridged biphenyl units in single molecular conductance measurementsGantenbein, M., Wang, L., Al-jobory, A., Ismael, A., Lambert, C., Hong, W., & Bryce, M. (2017). Quantum interference and heteroaromaticity of para- and meta-linked bridged biphenyl units in single molecular conductance measurements. Scientific Reports, 7, Article 1794. https://doi.org/10.1038/s41598-017-01903-0
- Color Tuning of Efficient Electroluminescence in the Blue and Green Regions Using Heteroleptic Iridium Complexes with 2-Phenoxyoxazole Ancillary LigandsBenjamin, H., Liang, J., Liu, Y., Geng, Y., Liu, X., Zhu, D., Batsanov, A. S., & Bryce, M. R. (2017). Color Tuning of Efficient Electroluminescence in the Blue and Green Regions Using Heteroleptic Iridium Complexes with 2-Phenoxyoxazole Ancillary Ligands. Organometallics, 36(9), 1810-1821. https://doi.org/10.1021/acs.organomet.7b00161
- Formation of Two-Dimensional Micelles on Graphene: Multi-Scale Theoretical and Experimental StudyRobinson, B. J., Bailey, S. W., O’Driscoll, L. J., Visontai, D., Welsh, D. J., Mostert, A. B., Mazzocco, R., Rabot, C., Jarvis, S. P., Kolosov, O. V., Bryce, M. R., & Lambert, C. (2017). Formation of Two-Dimensional Micelles on Graphene: Multi-Scale Theoretical and Experimental Study. ACS Nano, 11(3), 3404-3412. https://doi.org/10.1021/acsnano.7b01071
- A neutral dinuclear Ir(III) complex for anti-counterfeiting and data encryptionJiang, Y., Li, G., Che, W., Liu, Y., Xu, B., Shan, G., Zhu, D., Su, Z., & Bryce, M. (2017). A neutral dinuclear Ir(III) complex for anti-counterfeiting and data encryption. Chemical Communications, 53(21), 3022-3035. https://doi.org/10.1039/c7cc00769h
- Synthesis, Diastereomer Separation, and Optoelectronic and Structural Properties of Dinuclear Cyclometalated Iridium(III) Complexes with Bridging Diarylhydrazide LigandsCongrave, D. G., Hsu, Y., Batsanov, A. S., Beeby, A., & Bryce, M. R. (2017). Synthesis, Diastereomer Separation, and Optoelectronic and Structural Properties of Dinuclear Cyclometalated Iridium(III) Complexes with Bridging Diarylhydrazide Ligands. Organometallics, 36(5), 981-993. https://doi.org/10.1021/acs.organomet.6b00887
- The HOF structures of nitrotetraphenylethene derivatives provide new insights into the nature of AIE and a way to design mechanoluminescent materialsYu, T., Ou, D., Yang, Z., Huang, Q., Mao, Z., Chen, J., Zhang, Y., Liu, S., Xu, J., Bryce, M. R., & Chi, Z. (2017). The HOF structures of nitrotetraphenylethene derivatives provide new insights into the nature of AIE and a way to design mechanoluminescent materials. Chemical Science, 8(2), 1163-1168. https://doi.org/10.1039/c6sc03177c
- Bright Green PhOLEDs Using Cyclometalated Diiridium(III) Complexes with Bridging Oxamidato Ligands as Phosphorescent DopantsM’hamedi, A., Fox, M. A., Batsanov, A. S., Al-Attar, H. A., Monkman, A. P., & Bryce, M. R. (2017). Bright Green PhOLEDs Using Cyclometalated Diiridium(III) Complexes with Bridging Oxamidato Ligands as Phosphorescent Dopants. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 5(27), 6777-6789. https://doi.org/10.1039/c7tc00628d
- Rational design and characterization of heteroleptic phosphorescent iridium(III) complexes for highly efficient deep-blue OLEDsFeng, Y., Zhuang, X., Zhu, D., Liu, Y., Wang, Y., & Bryce, M. R. (2016). Rational design and characterization of heteroleptic phosphorescent iridium(III) complexes for highly efficient deep-blue OLEDs. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 4(43), 10246-10252. https://doi.org/10.1039/c6tc04119a
- Experimental and Computational Studies of Single-Molecule Conductance of Ru(II) and Pt(II) trans-Bis(acetylide) ComplexesAl-Owaedi, O. A., Milan, D. C., Oerthel, M., Bock, S., Yufit, D. S., Howard, J. A., Higgins, S. J., Nichols, R. J., Lambert, C. J., Bryce, M. R., & Low, P. J. (2016). Experimental and Computational Studies of Single-Molecule Conductance of Ru(II) and Pt(II) trans-Bis(acetylide) Complexes. Organometallics, 35(17), 2944-2954. https://doi.org/10.1021/acs.organomet.6b00472
- Sulfonyl-Substituted Heteroleptic Cyclometalated Iridium(III) Complexes as Blue Emitters for Solution-Processable Phosphorescent Organic Light-Emitting DiodesBenjamin, H., Zheng, Y., Batsanov, A., Fox, M., Al-Attar, H., Monkman, A., & Bryce, M. (2016). Sulfonyl-Substituted Heteroleptic Cyclometalated Iridium(III) Complexes as Blue Emitters for Solution-Processable Phosphorescent Organic Light-Emitting Diodes. Inorganic Chemistry, 55(17), 8612-8627. https://doi.org/10.1021/acs.inorgchem.6b01179
- Using Guest–Host Interactions To Optimize the Efficiency of TADF OLEDsdos Santos, P. L., Ward, J. S., Bryce, M. R., & Monkman, A. P. (2016). Using Guest–Host Interactions To Optimize the Efficiency of TADF OLEDs. Journal of Physical Chemistry Letters, 2016(7), 3341-3346. https://doi.org/10.1021/acs.jpclett.6b01542
- Pendant Homopolymer and Copolymers as Solution-Processable Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting DiodesRen, Z., Nobuyasu, R. S., Dias, F. B., Monkman, A. P., Yan, S., & Bryce, M. R. (2016). Pendant Homopolymer and Copolymers as Solution-Processable Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Macromolecules, 49(15), 5452-5460. https://doi.org/10.1021/acs.macromol.6b01216
- Achieving Very Bright Mechanoluminescence from Purely Organic Luminophores with Aggregation-Induced Emission by Crystal DesignXu, B., Li, W., He, J., Wu, S., Zhu, Q., Yang, Z., Wu, W., Zhang, Y., Jin, C., Lu, P., Chi, Z., Liu, S., Xu, J., & Bryce, M. R. (2016). Achieving Very Bright Mechanoluminescence from Purely Organic Luminophores with Aggregation-Induced Emission by Crystal Design. Chemical Science, 7(8), 5307-5312. https://doi.org/10.1039/c6sc01325b
- Combined Aggregation Induced Emission (AIE), Photochromism and Photoresponsive Wettability in Simple Dichloro-substituted Triphenylethylene DerivativesOu, D., Yu, T., Yang, Z., Luan, T., Mao, Z., Zhang, Y., Liu, S., Xu, J., Chi, Z., & Bryce, M. R. (2016). Combined Aggregation Induced Emission (AIE), Photochromism and Photoresponsive Wettability in Simple Dichloro-substituted Triphenylethylene Derivatives. Chemical Science, 7(8), 5302-5306. https://doi.org/10.1039/c6sc01205a
- Solvent Dependence of the Single Molecule Conductance of Oligoyne-Based Molecular WiresMilan, D. C., Al-Owaedi, O. A., Oerthel, M., Marqués-González, S., Brooke, R. J., Bryce, M. R., Cea, P., Ferrer, J., Higgins, S. J., Lambert, C. J., Low, P. J., Zsolt Manrique, D., Martin, S., Nichols, R. J., Schwarzacher, W., & García-Suárez, V. M. (2016). Solvent Dependence of the Single Molecule Conductance of Oligoyne-Based Molecular Wires. Journal of Physical Chemistry C, 120(29), 15666-15674. https://doi.org/10.1021/acs.jpcc.5b08877
- Novel Emitting System Based on a Multifunctional Bipolar Phosphor: An Effective Approach for Highly Efficient Warm- White Light-Emitting Devices with High Color-Rendering Index at High LuminanceDu, X., Feng, Y., Zhu, D., Peng, T., Liu, Y., Wang, Y., & Bryce, M. (2016). Novel Emitting System Based on a Multifunctional Bipolar Phosphor: An Effective Approach for Highly Efficient Warm- White Light-Emitting Devices with High Color-Rendering Index at High Luminance. Advanced Materials, 28(8), 5963-5968. https://doi.org/10.1002/adma.201600451
- Rational Design of TADF Polymers Using a Donor–Acceptor Monomer with Enhanced TADF Efficiency Induced by the Energy Alignment of Charge Transfer and Local Triplet Excited StatesNobuyasu, R. S., Ren, Z., Griffiths, G. C., Batsanov, A. S., Data, P., Yan, S., Monkman, A. P., Bryce, M. R., & Dias, F. B. (2016). Rational Design of TADF Polymers Using a Donor–Acceptor Monomer with Enhanced TADF Efficiency Induced by the Energy Alignment of Charge Transfer and Local Triplet Excited States. Advanced Optical Materials, 4(4), 597-607. https://doi.org/10.1002/adom.201500689
- Achieving Remarkable Mechanochromism and White-Light Emission with Thermally Activated Delayed Fluorescence through the Molecular Heredity PrincipleXu, B., Mu, Y., Mao, Z., Xie, Z., Wu, H., Zhang, Y., Jin, C., Chi, Z., Liu, S., Xu, J., Wu, Y., Lu, P., Lien, A., & Bryce, M. (2016). Achieving Remarkable Mechanochromism and White-Light Emission with Thermally Activated Delayed Fluorescence through the Molecular Heredity Principle. Chemical Science, 7(3), 2201-2206. https://doi.org/10.1039/c5sc04155d
- The interplay of thermally activated delayed fluorescence (TADF) and room temperature organic phosphorescence in sterically-constrained donor–acceptor charge-transfer moleculesWard, J. S., Nobuyasu, R. S., Batsanov, A. S., Data, P., Monkman, A. P., Dias, F. B., & Bryce, M. R. (2016). The interplay of thermally activated delayed fluorescence (TADF) and room temperature organic phosphorescence in sterically-constrained donor–acceptor charge-transfer molecules. Chemical Communications, 52(12), 2612-2615. https://doi.org/10.1039/c5cc09645f
- Intermolecular Electronic Coupling of Organic Units for Efficient Persistent Room-Temperature PhosphorescenceYang, Z., Mao, Z., Zhang, X., Ou, D., Mu, Y., Zhang, Y., Zhao, C., Liu, S., Chi, Z., Xu, J., Wu, Y., Lu, P., Lien, A., & Bryce, M. (2016). Intermolecular Electronic Coupling of Organic Units for Efficient Persistent Room-Temperature Phosphorescence. Angewandte Chemie International Edition, 55(6), 2181-2185. https://doi.org/10.1002/anie.201509224
- Solution-processed blue/deep blue and white phosphorescent organic light emitting diodes (PhOLEDs) hosted by a polysiloxane derivative with pendant mCP (1, 3-bis(9-carbazolyl)benzene)Sun, D., Zhou, X., Liu, J., Sun, X., Li, H., Ren, Z., Ma, D., Bryce, M., & Yan, S. (2015). Solution-processed blue/deep blue and white phosphorescent organic light emitting diodes (PhOLEDs) hosted by a polysiloxane derivative with pendant mCP (1, 3-bis(9-carbazolyl)benzene). ACS Applied Materials and Interfaces, 7(51), 27989-27998. https://doi.org/10.1021/am507592s
- Anion-specific aggregation induced phosphorescence emission (AIPE) in an ionic iridium complex in aqueous mediaLi, G., Guan, W., Du, S., Zhu, D., Shan, G., Zhu, X., Yan, L., Su, Z., Bryce, M. R., & Monkman, A. P. (2015). Anion-specific aggregation induced phosphorescence emission (AIPE) in an ionic iridium complex in aqueous media. Chemical Communications, 51(95), 16924-16927. https://doi.org/10.1039/c5cc07187a
- Key role of the linker in pyrene-linker-carboxylate surfactants for the efficient aqueous dispersion of multiwalled carbon nanotubesWelsh, D. J., O’Driscoll, L. J., Bailey, S. W., Visontai, D., Howes, K., Frampton, H., Bryce, M. R., & Lambert, C. J. (2015). Key role of the linker in pyrene-linker-carboxylate surfactants for the efficient aqueous dispersion of multiwalled carbon nanotubes. RSC Advances, 5(115), 95360-95368. https://doi.org/10.1039/c5ra20250g
- High brightness deep blue/violet fluorescent polymer light-emitting diodes (PLEDs)Cook, J. H., Santos, J., Al-Attar, H. A., Bryce, M. R., & Monkman, A. P. (2015). High brightness deep blue/violet fluorescent polymer light-emitting diodes (PLEDs). Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 3(37), 9664-9669. https://doi.org/10.1039/c5tc02162f
- Arylsilanes and siloxanes as optoelectronic materials for organic light-emitting diodes (OLEDs)Sun, D., Ren, Z., Bryce, M. R., & Yan, S. (2015). Arylsilanes and siloxanes as optoelectronic materials for organic light-emitting diodes (OLEDs). Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 3(37), 9496-9508. https://doi.org/10.1039/c5tc01638j
- Syntheses and Structures of Buta-1,3-Diynyl Complexes from ‘On Complex’ Cross-Coupling ReactionsOerthel, M., Yufit, D., Fox, M., Bryce, M., & Low, P. (2015). Syntheses and Structures of Buta-1,3-Diynyl Complexes from ‘On Complex’ Cross-Coupling Reactions. Organometallics, 34(11), 2395-2405. https://doi.org/10.1021/om501186c
- The role of exciplex states in phosphorescent OLEDs with poly(vinylcarbazole) (PVK) hostJankus, V., Abdullah, K., Griffiths, G. C., Al-Attar, H., Zheng, Y., Bryce, M. R., & Monkman, A. P. (2015). The role of exciplex states in phosphorescent OLEDs with poly(vinylcarbazole) (PVK) host. Organic Electronics, 20, 97-102. https://doi.org/10.1016/j.orgel.2015.02.002
- Fluorene Co-polymers with High Efficiency Deep-Blue ElectroluminescenceSantos, J., Cook, J., Al-Attar, H., Monkman, A., & Bryce, M. (2015). Fluorene Co-polymers with High Efficiency Deep-Blue Electroluminescence. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 3(11), 2479-2483. https://doi.org/10.1039/c4tc02766c
- Correlation of breaking forces, conductances and geometries of molecular junctionsYoshida, K., Pobelov, I. V., Manrique, D. Z., Pope, T., Mészáros, G., Gulcur, M., Bryce, M. R., Lambert, C. J., & Wandlowski, T. (2015). Correlation of breaking forces, conductances and geometries of molecular junctions. Scientific Reports, 5. https://doi.org/10.1038/srep09002
- Oligo(p-phenyleneethynylene) (OPE) Molecular Wires: Synthesis and Length Dependence of Photoinduced Charge Transfer in OPEs with Triarylamine and Diaryloxadiazole End GroupsLinton, K., Fox, M., Pålsson, L., & Bryce, M. (2015). Oligo(p-phenyleneethynylene) (OPE) Molecular Wires: Synthesis and Length Dependence of Photoinduced Charge Transfer in OPEs with Triarylamine and Diaryloxadiazole End Groups. Chemistry - A European Journal, 21(10), 3997-4007. https://doi.org/10.1002/chem.201406080
- A quantum circuit rule for interference effects in single-molecule electrical junctionsManrique, D. Z., Huang, C., Baghernejad, M., Zhao, X., Al-Owaedi, O. A., Sadeghi, H., Kaliginedi, V., Hong, W., Gulcur, M., Wandlowski, T., Bryce, M. R., & Lambert, C. J. (2015). A quantum circuit rule for interference effects in single-molecule electrical junctions. Nature Communications, 6, Article 6389. https://doi.org/10.1038/ncomms7389
- New AIE-active dinuclear Ir(III) complexes with reversible piezochromic phosphorescence behaviourLi, G., Ren, X., Shan, G., Che, W., Zhu, D., Yan, L., Su, Z., & Bryce, M. R. (2015). New AIE-active dinuclear Ir(III) complexes with reversible piezochromic phosphorescence behaviour. Chemical Communications, 51(65), 13036-13039. https://doi.org/10.1039/c5cc04850h
- Very High Efficiency Orange-Red Light-Emitting Devices with Low Roll-Off at High Luminance Based on an Ideal Host-Guest System Consisting of Two Novel Phosphorescent Iridium Complexes with Bipolar TransportLi, G., Zhu, D., Peng, T., Liu, Y., Wang, Y., & Bryce, M. R. (2014). Very High Efficiency Orange-Red Light-Emitting Devices with Low Roll-Off at High Luminance Based on an Ideal Host-Guest System Consisting of Two Novel Phosphorescent Iridium Complexes with Bipolar Transport. Advanced Functional Materials, 24(47), 7420-7426. https://doi.org/10.1002/adfm.201402177
- Electrochemical control of single-molecule conductance by Fermi-level tuning and conjugation switchingBaghernejad, M., Zhao, X., Ørnsø, K., Füeg, M., Moreno-García, P., Rudnev, A., Kaliginedi, V., Vesztergom, S., Huang, C., Hong, W., Broekmann, P., Wandlowski, T., Thygesen, K., & Bryce, M. (2014). Electrochemical control of single-molecule conductance by Fermi-level tuning and conjugation switching. Journal of the American Chemical Society, 136(52), 17922-17925. https://doi.org/10.1021/ja510335z
- Highly Efficient TADF OLEDs: How the Emitter–Host Interaction Controls Both the Excited State Species and Electrical Properties of the Devices to Achieve Near 100% Triplet Harvesting and High EfficiencyJankus, V., Data, P., Graves, D., McGuinness, C., Santos, J., Bryce, M., Dias, F., & Monkman, A. (2014). Highly Efficient TADF OLEDs: How the Emitter–Host Interaction Controls Both the Excited State Species and Electrical Properties of the Devices to Achieve Near 100% Triplet Harvesting and High Efficiency. Advanced Functional Materials, 24(39), 6178-6186. https://doi.org/10.1002/adfm.201400948
- A versatile hybrid polyphenylsilane host for highly efficient solution-processed blue and deep blue electrophosphorescenceSun, D., Zhou, X., Li, H., Sun, X., Zheng, Y., Ren, Z., Ma, D., Bryce, M. R., & Yan, S. (2014). A versatile hybrid polyphenylsilane host for highly efficient solution-processed blue and deep blue electrophosphorescence. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 2(39), 8277-8284. https://doi.org/10.1039/c4tc01467g
- Bimetallic Cyclometalated Iridium(III) Diastereomers with Non-Innocent Bridging Ligands for High-Efficiency Phosphorescent OLEDsZheng, Y., Batsanov, A., Fox, M., Al-Attar, H., Abdullah, K., Jankus, V., Bryce, M., & Monkman, A. (2014). Bimetallic Cyclometalated Iridium(III) Diastereomers with Non-Innocent Bridging Ligands for High-Efficiency Phosphorescent OLEDs. Angewandte Chemie International Edition, 53(43), 11616-11619. https://doi.org/10.1002/anie.201407475
- Structural versus Electrical Functionalization of Oligo(phenyleneethynylene) Diamine Molecular JunctionsGonzález, M. T., Zhao, X., Manrique, D. Z., Miguel, D., Leary, E., Gulcur, M., Batsanov, A. S., Rubio-Bollinger, G., Lambert, C. J., Bryce, M. R., & Agraït, N. (2014). Structural versus Electrical Functionalization of Oligo(phenyleneethynylene) Diamine Molecular Junctions. Journal of Physical Chemistry C, 118(37), 21655-21662. https://doi.org/10.1021/jp506078a
- Anisotropic highly-conductive films of poly(3-methylthiophene) from epitaxial electropolymerization on oriented poly(vinylidene fluoride)Sun, D., Li, Y., Ren, Z., Bryce, M. R., Li, H., & Yan, S. (2014). Anisotropic highly-conductive films of poly(3-methylthiophene) from epitaxial electropolymerization on oriented poly(vinylidene fluoride). Chemical Science, 5(8), 3240-3245. https://doi.org/10.1039/c4sc01068j
- Efficient deep blue fluorescent polymer light-emitting diodes (PLEDs)Cook, J. H., Santos, J., Li, H., Al-Attar, H. A., Bryce, M. R., & Monkman, A. P. (2014). Efficient deep blue fluorescent polymer light-emitting diodes (PLEDs). Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 2(28), 5587-5592. https://doi.org/10.1039/c4tc00896k
- New ionic dinuclear Ir(III) Schiff base complexes with aggregation-induced phosphorescent emission (AIPE)Li, G., Wu, Y., Shan, G., Che, W., Zhu, D., Song, B., Yan, L., Su, Z., & Bryce, M. R. (2014). New ionic dinuclear Ir(III) Schiff base complexes with aggregation-induced phosphorescent emission (AIPE). Chemical Communications, 50(53), 6977-6980. https://doi.org/10.1039/c4cc01799d
- Intramolecular π Stacking in Cationic Iridium(III) Complexes with Phenyl-Functionalized Cyclometalated Ligands: Synthesis, Structure, Photophysical Properties, and Theoretical StudiesLi, P., Shan, G., Cao, H., Zhu, D., Su, Z., Jitchati, R., & Bryce, M. R. (2014). Intramolecular π Stacking in Cationic Iridium(III) Complexes with Phenyl-Functionalized Cyclometalated Ligands: Synthesis, Structure, Photophysical Properties, and Theoretical Studies. European Journal of Inorganic Chemistry, 2014(14), 2376-2382. https://doi.org/10.1002/ejic.201400007
- The Synthesis of Functionalised Diaryltetraynes and Their Transport Properties in Single-Molecule JunctionsGulcur, M., Moreno-García, P., Zhao, X., Baghernejad, M., Batsanov, A., Hong, W., Bryce, M., & Wandlowski, T. (2014). The Synthesis of Functionalised Diaryltetraynes and Their Transport Properties in Single-Molecule Junctions. Chemistry - A European Journal, 20(16), 4653-4660. https://doi.org/10.1002/chem.201304671
- A Carbazole-Oxadiazole Diad Molecule for Single-Emitting-Component White Organic Light-Emitting Devices (WOLEDs)Wu, X., Wang, L., Hua, Y., Wang, C., Batsanov, A. S., & Bryce, M. R. (2014). A Carbazole-Oxadiazole Diad Molecule for Single-Emitting-Component White Organic Light-Emitting Devices (WOLEDs). Tetrahedron, 70(11), 2015-2019. https://doi.org/10.1016/j.tet.2014.01.073
- A study of planar anchor groups for graphene-based single-molecule electronicsBailey, S., Visontai, D., Lambert, C. J., Bryce, M. R., Frampton, H., & Chappell, D. (2014). A study of planar anchor groups for graphene-based single-molecule electronics. Journal of Chemical Physics, 140(5), Article 054708. https://doi.org/10.1063/1.4861941
- Oligo(aryleneethynylene)s with Terminal Pyridyl Groups: Synthesis and Length Dependence of the Tunnelling to Hopping Transition in Single-Molecule ConductancesZhao, X., Huang, C., Gulcur, M., Batsanov, A. S., Baghernejad, M., Hong, W., Bryce, M. R., & Wandlowski, T. (2013). Oligo(aryleneethynylene)s with Terminal Pyridyl Groups: Synthesis and Length Dependence of the Tunnelling to Hopping Transition in Single-Molecule Conductances. Chemistry of Materials, 25(21), 4340-4347. https://doi.org/10.1021/cm4029484
- New Oxazoline- and Thiazoline-Containing Heteroleptic Iridium(III) Complexes for Highly-Efficient Phosphorescent Organic Light-Emitting Devices (PhOLEDs): Colour Tuning by Varying the Electroluminescence BandwidthChao, K., Shao, K., Peng, T., Zhu, D., Wang, Y., Liu, Y., Sua, Z., & Bryce, M. R. (2013). New Oxazoline- and Thiazoline-Containing Heteroleptic Iridium(III) Complexes for Highly-Efficient Phosphorescent Organic Light-Emitting Devices (PhOLEDs): Colour Tuning by Varying the Electroluminescence Bandwidth. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 1(41), 6800-6806. https://doi.org/10.1039/c3tc31463d
- Efficient light-emitting electrochemical cells (LECs) based on ionic iridium(III) complexes with 1,3,4-oxadiazole ligands.Zhang, J., Zhou, L., Al-Attar, H., Shao, K., Wang, L., Zhu., D., Su, Z., Bryce, M., & Monkman, A. (2013). Efficient light-emitting electrochemical cells (LECs) based on ionic iridium(III) complexes with 1,3,4-oxadiazole ligands. Advanced Functional Materials, 23(37), 4667-4677. https://doi.org/10.1002/adfm.201300344
- Single-Molecule Conductance of Functionalized Oligoynes: Length Dependence and Junction EvolutionMoreno-García, P., Gulcur, M., Manrique, D., Pope, T., Hong, W., Kaliginedi, V., Huang, C., Batsanov, A., Bryce, M., Lambert, C., & Wandlowski, T. (2013). Single-Molecule Conductance of Functionalized Oligoynes: Length Dependence and Junction Evolution. Journal of the American Chemical Society, 135(33), 12228-12240. https://doi.org/10.1021/ja4015293
- Triplet Harvesting with 100% Efficiency by Way of Thermally Activated Delayed Fluorescence in Charge Transfer OLED Emitters.Dias, F., Bourdakos, K., Jankus, V., Moss, K., Kamtekar, K., Bhalla, V., Santos, J., Bryce, M., & Monkman, A. (2013). Triplet Harvesting with 100% Efficiency by Way of Thermally Activated Delayed Fluorescence in Charge Transfer OLED Emitters. Advanced Materials, 25(27), 3707-3714. https://doi.org/10.1002/adma.201300753
- Cyclometalated Ir(III) Complexes for High-Efficiency Solution-Processable Blue PhOLEDsKozhevnikov, V. N., Zheng, Y., Clough, M., Al-Attar, H. A., Griffiths, G. C., Abdullah, K., Raisys, S., Jankus, V., Bryce, M. R., & Monkman, A. P. (2013). Cyclometalated Ir(III) Complexes for High-Efficiency Solution-Processable Blue PhOLEDs. Chemistry of Materials, 25(11), 2352-2358. https://doi.org/10.1021/cm4010773
- Unambiguous One-Molecule Conductance Measurements under Ambient ConditionsLeary, E., González, M., van der Pol, C., Bryce, M., Filippone, S., Martín, N., Rubio-Bollinger, G., & Agraït, N. (2011). Unambiguous One-Molecule Conductance Measurements under Ambient Conditions. Nano Letters, 11(6). https://doi.org/10.1021/nl200294s
- Molecular Bridging of Silicon NanogapsAshwell, G., Phillips, L., Robinson, B., Urasinska-Wojcik, B., Lambert, C., Grace, I., Bryce, M., Jitchati, R., Tavasli, M., Cox, T., Sage, I., Tuffin, R., & Ray, S. (2010). Molecular Bridging of Silicon Nanogaps. ACS Nano, 4(12). https://doi.org/10.1021/nn102460z
- Identifying Diversity in Nanoscale Electrical Break JunctionsMartín, S., Grace, I., Bryce, M., Wang, C., Jitchati, R., Batsanov, A., Higgins, S., Lambert, C., & Nichols, R. (2010). Identifying Diversity in Nanoscale Electrical Break Junctions. Journal of the American Chemical Society, 132(26), 9157-9164. https://doi.org/10.1021/ja103327f
- Efficient Intramolecular Charge Transfer in Oligoyne-Linked Donor–π–Acceptor MoleculesPålsson, L., Wang, C., Batsanov, A., King, S., Beeby, A., Monkman, A., & Bryce, M. (2009). Efficient Intramolecular Charge Transfer in Oligoyne-Linked Donor–π–Acceptor Molecules. Chemistry - A European Journal, 16(5), 1470-1479. https://doi.org/10.1002/chem.200902099
- Electrical Conductance of Conjugated Oligomers at the Single Molecule LevelHuber, R., González, M., Wu, S., Langer, M., Grunder, S., Horhoiu, V., Mayor, M., Bryce, M., Wang, C., Jitchati, R., Schönenberger, C., & Calame, M. (2008). Electrical Conductance of Conjugated Oligomers at the Single Molecule Level. Journal of the American Chemical Society, 130(3), 1080-1084. https://doi.org/10.1021/ja0767940
- Calix 6 arene derivatives selectively functionalized at alternate sites on the smaller rim with 2-phenylpyridine and 2-fluorenylpyridine substituents to provide deep cavitiesZeng, X., Batsanov, A., & Bryce, M. (2006). Calix 6 arene derivatives selectively functionalized at alternate sites on the smaller rim with 2-phenylpyridine and 2-fluorenylpyridine substituents to provide deep cavities. Journal of Organic Chemistry, 71(26), 9589-9594. https://doi.org/10.1021/jo0614341
- Convergent synthesis of 10 nm aryleethynylene molecular wires by an iterative regioselective deprotection/sonogashira coupling protocolWang, C., Batsanov, A., & Bryce, M. (2006). Convergent synthesis of 10 nm aryleethynylene molecular wires by an iterative regioselective deprotection/sonogashira coupling protocol. Journal of Organic Chemistry, 71(1), 108-116. https://doi.org/10.1021/jo051711o
- Synthesis of new axially-disubstituted silicon-phthalocyanine derivatives : optical and structural characterisation.Barker, C., Findlay, K., Bettington, S., Batsanov, A., Perepichka, I., Bryce, M., & Beeby, A. (2006). Synthesis of new axially-disubstituted silicon-phthalocyanine derivatives : optical and structural characterisation. Tetrahedron, 62(40), 9433-9439. https://doi.org/10.1016/j.tet.2006.07.046
- Single-molecule electrical studies on a 7 nm long molecular wire.Ashwell, G., Urasinska, B., Wang, C., Bryce, M., Grace, I., & Lambert, C. (2006). Single-molecule electrical studies on a 7 nm long molecular wire. Chemical Communications, 2006(45), 4706-4708. https://doi.org/10.1039/b613347a
- Intramolecular charge transfer assisted by conformational changes in the excited state of fluorene-dibenzothiophene-S,S-dioxide co-oligomersDias, F., Pollock, S., Hedley, G., Palsson, L., Monkman, A., Perepichka, I., Perepichka, I., Tavasli, M., & Bryce, M. (2006). Intramolecular charge transfer assisted by conformational changes in the excited state of fluorene-dibenzothiophene-S,S-dioxide co-oligomers. Journal of Physical Chemistry B (Soft Condensed Matter and Biophysical Chemistry), 110(39), 19329-19339. https://doi.org/10.1021/jp0643653
- Extreme conformational constraints in pi-extended tetrathiafulvalenes : Unusual topologies and redox behavior of doubly and triply bridged cyclophanes.Christensen, C., Batsanov, A., & Bryce, M. (2006). Extreme conformational constraints in pi-extended tetrathiafulvalenes : Unusual topologies and redox behavior of doubly and triply bridged cyclophanes. Journal of the American Chemical Society, 128(32), 10484-10490. https://doi.org/10.1021/ja062358m
- Nanoscale aryleneethynylene oligomers incorporating fluorenone units as electron-dopable molecular wires.Wang, C., Batsanov, A., & Bryce, M. (2006). Nanoscale aryleneethynylene oligomers incorporating fluorenone units as electron-dopable molecular wires. Faraday Discussions, 131, 221-234. https://doi.org/10.1039/b506712j
- Remarkable interplay of redox states and conformational changes in a sterically crowded, cross-conjugated tetrathiafulvalene vinylog.Amriou, S., Perepichka, I., Batsanov, A., Bryce, M., Rovira, C., & Vidal-Gancedo, J. (2006). Remarkable interplay of redox states and conformational changes in a sterically crowded, cross-conjugated tetrathiafulvalene vinylog. Chemistry - A European Journal, 12(21), 5481-5494. https://doi.org/10.1002/chem.200600244
- The interplay of inverted redox potentials and aromaticity in the oxidized states of new pi-electron donors: 9-(1,3-dithiol-2-ylidene)fluorene and 9-(1,3-dithiol-2-ylidene)thioxanthene derivatives.Amriou, S., Wang, C., Batsanov, A., Bryce, M., Perepichka, D., Orti, E., Viruela, R., Vidal-Gancedo, J., & Rovira, C. (2006). The interplay of inverted redox potentials and aromaticity in the oxidized states of new pi-electron donors: 9-(1,3-dithiol-2-ylidene)fluorene and 9-(1,3-dithiol-2-ylidene)thioxanthene derivatives. Chemistry - A European Journal, 12(12), 3389-3400. https://doi.org/10.1002/chem.200501326
- Electronic interactions in a new pi-extended tetrathiafulvalene dimer.Diaz, M., Illescas, B., Martin, N., Perepichka, I., Bryce, M., Levillain, E., Viruela, R., & Orti, E. (2006). Electronic interactions in a new pi-extended tetrathiafulvalene dimer. Chemistry - A European Journal, 12(10), 2709-2721. https://doi.org/10.1002/chem.200501001
- Organic rectifying junctions from an electron-accepting molecular wire and an electron-donating phthalocyanine.Ashwell, G., Tyrrell, W., Urasinska, B., Wang, C., & Bryce, M. (2006). Organic rectifying junctions from an electron-accepting molecular wire and an electron-donating phthalocyanine. Chemical Communications, 2006(15), 1640-1642. https://doi.org/10.1039/b600617e
- Bridged diiridium complexes for electrophosphorescent OLEDs : synthesis, X-ray crystal structures, photophysics, and devices.Bettington, S., Tavasli, M., Bryce, M., Batsanov, A., Thompson, A., Al Attar, H., Dias, F., & Monkman, A. (2006). Bridged diiridium complexes for electrophosphorescent OLEDs : synthesis, X-ray crystal structures, photophysics, and devices. Journal of Materials Chemistry, 16(11), 1046-1052. https://doi.org/10.1039/b515258e
- Precision control of single-molecule electrical junctionsHaiss, W., Wang, C., Grace, I., Batsanov, A., Schiffrin, D., Higgins, S., Bryce, M., Lambert, C., & Nichols, R. (2006). Precision control of single-molecule electrical junctions. Nature Materials, 5(12), 995-1002. https://doi.org/10.1038/nmat1781
- New electroluminescent bipolar compounds for balanced charge-transport and tuneable colour in organic light emitting diodes: triphenylamine oxadiazole-fluorene triad moleculesKamtekar, K., Wang, C., Bettington, S., Batsanov, A., Perepichka, I., Bryce, M., Ahn, J., Rabinal, M., & Petty, M. (2006). New electroluminescent bipolar compounds for balanced charge-transport and tuneable colour in organic light emitting diodes: triphenylamine oxadiazole-fluorene triad molecules. Journal of Materials Chemistry, 16(39), 3823-3835. https://doi.org/10.1039/b604543j
- Are terminal aryl butadiynes stable? Synthesis and X-ray crystal structures of a series of aryl- and heteroaryl-butadiynes (Ar-C C-C C-H)West, K., Wang, C., Batsanov, A., & Bryce, M. (2006). Are terminal aryl butadiynes stable? Synthesis and X-ray crystal structures of a series of aryl- and heteroaryl-butadiynes (Ar-C C-C C-H). Journal of Organic Chemistry, 71(22), 8541-8544. https://doi.org/10.1021/jo0615697
- Self-assembly and multistage redox chemistry of strong electron acceptors on metal surfaces: Polynitrofluorenes on gold and platinumPerepichka, D., Kondratenko, M., & Bryce, M. (2005). Self-assembly and multistage redox chemistry of strong electron acceptors on metal surfaces: Polynitrofluorenes on gold and platinum. Langmuir, 21(19), 8824-8831.
- Thermal annealing of blended-layer organic light-emitting diodesAhn, J., Wang, C., Widdowson, N., Pearson, C., Bryce, M., & Petty, M. (2005). Thermal annealing of blended-layer organic light-emitting diodes. Journal of Applied Physics, 98(5).
- The first studies of a tetrathiafulvalene-sigma-acceptor molecular rectifierHo, G., Heath, J., Kondratenko, M., Perepichka, D., Arseneault, K., Pezolet, M., & Bryce, M. (2005). The first studies of a tetrathiafulvalene-sigma-acceptor molecular rectifier. Chemistry - A European Journal, 11(10), 2914-2922.
- 2-Ethoxy-3-pyridylboronic acid: A versatile reagent for the synthesis of highly-functionalised 3-aryl/heteroaryl-pyridines via Suzuki cross-coupling reactionsThompson, A., Batsanov, A., Bryce, M., Saygili, N., Parry, P., & Tarbit, B. (2005). 2-Ethoxy-3-pyridylboronic acid: A versatile reagent for the synthesis of highly-functionalised 3-aryl/heteroaryl-pyridines via Suzuki cross-coupling reactions. Tetrahedron, 61(21), 5131-5135.
- White polymeric light-emitting diode based on a fluorene polymer/Ir complex blend systemAl Attar, H., Monkman, A., Tavasli, M., Bettington, S., & Bryce, M. (2005). White polymeric light-emitting diode based on a fluorene polymer/Ir complex blend system. Applied Physics Letters, 86(12).
- Oligo(fluorenyl)pyridine ligands and their iridium complexes: synthesis, photophysical properties and electrophosphorescent devicesTavasli, M., Bettington, S., Bryce, M., Al Attar, H., Dias, F., King, S., & Monkman, A. (2005). Oligo(fluorenyl)pyridine ligands and their iridium complexes: synthesis, photophysical properties and electrophosphorescent devices. Journal of Materials Chemistry, 15, 4963-4970.
- Palladium-catalyzed cross-coupling reactions of pyridylboronic acids with heteroaryl halides bearing a primary amine group: Synthesis of highly substituted bipyridines and pyrazinopyridinesThompson, A., Hughes, G., Batsanov, A., Bryce, M., Parry, P., & Tarbit, B. (2005). Palladium-catalyzed cross-coupling reactions of pyridylboronic acids with heteroaryl halides bearing a primary amine group: Synthesis of highly substituted bipyridines and pyrazinopyridines. Journal of Organic Chemistry, 70(1), 388-390.
- Dibenzothiophene-S,S-dioxide-fluorene co-oligomers. Stable, highly-efficient blue emitters with improved electron affinityPerepichka, I., Perepichka, I., Bryce, M., & Palsson, L. (2005). Dibenzothiophene-S,S-dioxide-fluorene co-oligomers. Stable, highly-efficient blue emitters with improved electron affinity. Chemical Communications, 2005(27), 3397-3399. https://doi.org/10.1039/B417717G
- Electron-transporting materials for organic electroluminescent and electrophosphorescent devicesHughes, G., & Bryce, M. (2005). Electron-transporting materials for organic electroluminescent and electrophosphorescent devices. Journal of Materials Chemistry, 15(1), 94-107.
- A versatile synthesis of pyrazolo 3,4-c isoquinoline derivatives by reaction of 4-aryl-5-aminopyrazoles with aryl/heteroaryl aldehydes: the effect of the heterocycle on the reaction pathwaysBogza, S., Kobrakov, K., Malienko, A., Perepichka, I., Sujkov, S., Bryce, M., Lyubchik, S., Batsanov, A., & Bogdan, N. (2005). A versatile synthesis of pyrazolo 3,4-c isoquinoline derivatives by reaction of 4-aryl-5-aminopyrazoles with aryl/heteroaryl aldehydes: the effect of the heterocycle on the reaction pathways. Organic and Biomolecular Chemistry, 3(5), 932-940.
- New 2,5-diaryl-1,3,4-oxadiazole-fluorene hybrids as electron transporting materials for blended-layer organic light emitting diodesOyston, S., Wang, C., Hughes, G., Batsanov, A., Perepichka, I., Bryce, M., Ahn, J., Pearson, C., & Petty, M. (2005). New 2,5-diaryl-1,3,4-oxadiazole-fluorene hybrids as electron transporting materials for blended-layer organic light emitting diodes. Journal of Materials Chemistry, 15(1), 194-203.
- Functionalised 9-(1,3-dithiol-2-ylidene)thioxanthene derivatives: a C-60 conjugate as an ambipolar organic field effect transistor (OFET)Amriou, S., Mehta, A., & Bryce, M. (2005). Functionalised 9-(1,3-dithiol-2-ylidene)thioxanthene derivatives: a C-60 conjugate as an ambipolar organic field effect transistor (OFET). Journal of Materials Chemistry, 15(12), 1232-1234.
- Molecules with exceptionally small HOMO-LUMO gapsPerepichka, D., & Bryce, M. (2005). Molecules with exceptionally small HOMO-LUMO gaps. Angewandte Chemie International Edition, 44(34), 5370-5373.
- Organic light-emitting diodes based on a blend of poly 2-(2-ethylhexyloxy)-5-methoxy-1,4-phenylenevinylene and an electron transporting materialAhn, J., Wang, C., Pearson, C., Bryce, M., & Petty, M. (2004). Organic light-emitting diodes based on a blend of poly 2-(2-ethylhexyloxy)-5-methoxy-1,4-phenylenevinylene and an electron transporting material. Applied Physics Letters, 85(7), 1283-1285.
- Nanoscale aryleneethynylene molecular wires with reversible fluorenone electrochemistry for self-assembly onto metal surfacesWang, C., Batsanov, A., Bryce, M., & Sage, I. (2004). Nanoscale aryleneethynylene molecular wires with reversible fluorenone electrochemistry for self-assembly onto metal surfaces. Organic Letters, 6(13), 2181-2184.
- 5-Pyrimidylboronic acid and 2-methoxy-5-pyrimidylboronic acid: new heteroarylpyrimidine derivatives via Suzuki cross-coupling reactionsSaygili, N., Batsanov, A., & Bryce, M. (2004). 5-Pyrimidylboronic acid and 2-methoxy-5-pyrimidylboronic acid: new heteroarylpyrimidine derivatives via Suzuki cross-coupling reactions. Organic and Biomolecular Chemistry, 2(6), 852-857.
- Functionalisation reactions of 2,5-diphenyl-1,3,4-oxadiazoles bearing a terminal ethynyl or butadiynyl substituent: X-ray crystal structures of the productsKreher, D., Batsanov, A., Wang, C., & Bryce, M. (2004). Functionalisation reactions of 2,5-diphenyl-1,3,4-oxadiazoles bearing a terminal ethynyl or butadiynyl substituent: X-ray crystal structures of the products. Organic and Biomolecular Chemistry, 2(6), 858-862.
- Electrochromic tetrathiafulvalene derivatives functionalised with 2,5-diaryl-1,3,4-oxadiazole chromophoresWang, C., Batsanov, A., & Bryce, M. (2004). Electrochromic tetrathiafulvalene derivatives functionalised with 2,5-diaryl-1,3,4-oxadiazole chromophores. Chemical Communications, 2004(5), 578-579. https://doi.org/10.1039/B316243P
- 2,5-di(aryleneethynyl)pyrazine derivatives: synthesis, structural and optoelectronic properties, and light-emitting deviceZhao, L., Perepichka, I., Turksoy, F., Batsanov, A., Beeby, A., Findlay, K., & Bryce, M. (2004). 2,5-di(aryleneethynyl)pyrazine derivatives: synthesis, structural and optoelectronic properties, and light-emitting device. New Journal of Chemistry, 28(8), 912-918.
- Ethynyl pi-extended 2,5-diphenyl-1,3,4-oxadiazoles and 2-phenyl 5-(2-thienyl)-1,3,4-oxadiazoles: synthesis, X-ray crystal structures and optical propertiesHughes, G., Kreher, D., Wang, C., Batsanov, A., & Bryce, M. (2004). Ethynyl pi-extended 2,5-diphenyl-1,3,4-oxadiazoles and 2-phenyl 5-(2-thienyl)-1,3,4-oxadiazoles: synthesis, X-ray crystal structures and optical properties. Organic and Biomolecular Chemistry, 2(22), 3363-3367.
- A covalent tetrathiafulvalene-tetracyanoquinodimethane diad: Extremely low HOMO-LUMO gap, thermoexcited electron transfer, and high-quality Langmuir-Blodgett filmsPerepichka, D., Bryce, M., Pearson, C., Petty, M., McInnes, E., & Zhao, J. (2003). A covalent tetrathiafulvalene-tetracyanoquinodimethane diad: Extremely low HOMO-LUMO gap, thermoexcited electron transfer, and high-quality Langmuir-Blodgett films. Angewandte Chemie International Edition, 42(38), 4636-4639. https://doi.org/10.1002/anie.200351876
- Probing charge separation in structurally different C-60/exTTF ensemblesDiaz, M., Herranz, M., Illescas, B., Martin, N., Godbert, N., Bryce, M., Luo, C., Swartz, A., Anderson, G., & Guldi, D. (2003). Probing charge separation in structurally different C-60/exTTF ensembles. Journal of Organic Chemistry, 68(20), 7711-7721.
- Arborol-functionalised tetrathiafulvalene derivatives: Synthesis and thin-film formationLe Gall, T., Pearson, C., Bryce, M., Petty, M., Dahlgaard, H., & Becher, J. (2003). Arborol-functionalised tetrathiafulvalene derivatives: Synthesis and thin-film formation. European Journal of Organic Chemistry, 2003(18), 3562-3568. https://doi.org/10.1002/ejoc.200300286
- Planar chiral 2-ferrocenyloxazolines and 1,1 '-bis(oxazolinyl)ferrocenes-syntheses and applications in asymmetric catalysisSutcliffe, O., & Bryce, M. (2003). Planar chiral 2-ferrocenyloxazolines and 1,1 ’-bis(oxazolinyl)ferrocenes-syntheses and applications in asymmetric catalysis. Tetrahedron: Asymmetry, 14(16), 2297-2325.
- 5-Formyl-2-furylboronic acid as a versatile bifunctional reagent for the synthesis of pi-extended heteroarylfuran systemsParry, P., Bryce, M., & Tarbit, B. (2003). 5-Formyl-2-furylboronic acid as a versatile bifunctional reagent for the synthesis of pi-extended heteroarylfuran systems. Organic and Biomolecular Chemistry, 1(9), 1447-1449.
- New strategies and building blocks for functionalised 9,10-bis(1,3-dithiol-2-ylidene)-9,10-dihydroanthracene derivatives, including pyrrolo-annelated derivatives and pi-extended systems with intramolecular charge-transferChristensen, C., Bryce, M., Batsanov, A., & Becher, J. (2003). New strategies and building blocks for functionalised 9,10-bis(1,3-dithiol-2-ylidene)-9,10-dihydroanthracene derivatives, including pyrrolo-annelated derivatives and pi-extended systems with intramolecular charge-transfer. Organic and Biomolecular Chemistry, 1(3), 511-522.
- New pyrimidine- and fluorene-containing oligo(arylene)s : synthesis, crystal structures, optoelectronic properties and a theoretical study.Hughes, G., Wang, C., Batsanov, A., Fern, M., Frank, S., Bryce, M., Perepichka, I., Monkman, A., & Lyons, B. (2003). New pyrimidine- and fluorene-containing oligo(arylene)s : synthesis, crystal structures, optoelectronic properties and a theoretical study. Organic and Biomolecular Chemistry, 2003(17), 3069-3077. https://doi.org/10.1039/b305870k
- New shelf-stable halo- and alkoxy-substituted pyridylboronic acids and their Suzuki cross-coupling reactions to yield heteroarylpyridinesParry, P., Bryce, M., & Tarbit, B. (2003). New shelf-stable halo- and alkoxy-substituted pyridylboronic acids and their Suzuki cross-coupling reactions to yield heteroarylpyridines. Synthesis : Journal of Synthetic Organic Chemistry., 7, 1035-1038. https://doi.org/10.1055/s-2003-39158
- Phenylene-2,5-dimethylpyrazine co-oligomers: synthesis by Suzuki couplings, X-ray structures of neutral and diprotonated teraryl species and efficient blue emissionTurksoy, F., Hughes, G., Batsanov, A., & Bryce, M. (2003). Phenylene-2,5-dimethylpyrazine co-oligomers: synthesis by Suzuki couplings, X-ray structures of neutral and diprotonated teraryl species and efficient blue emission. Journal of Materials Chemistry, 13(7), 1554-1557.
- Synthesis of novel phthalocyanine-tetrathiafulvalene hybrids; Intramolecular fluorescence quenching related to molecular geometryFarren, C., Christensen, C., FitzGerald, S., Bryce, M., & Beeby, A. (2002). Synthesis of novel phthalocyanine-tetrathiafulvalene hybrids; Intramolecular fluorescence quenching related to molecular geometry. Journal of Organic Chemistry, 67(26), 9130-9139.
- Electrochemically controlled interactions between TTF-based dendrimers and an electron-rich oligomerBeeby, A., Bryce, M., Christensen, C., Cooke, G., Duclairoir, F., & Rotello, V. (2002). Electrochemically controlled interactions between TTF-based dendrimers and an electron-rich oligomer. Chemical Communications, 2002(24), 2950-2951. https://doi.org/10.1039/B209765F
- Engineering a remarkably low HOMO-LUMO gap by covalent linkage of a strong π-donor and a π-acceptor-tetrathiafulvalene-σ-polynitrofluorene diads: Their amphoteric redox behavior, electron transfer and spectroscopic propertiesPerepichka, D., Bryce, M., Batsanov, A., McInnes, E., Zhao, J., & Farley, R. (2002). Engineering a remarkably low HOMO-LUMO gap by covalent linkage of a strong π-donor and a π-acceptor-tetrathiafulvalene-σ-polynitrofluorene diads: Their amphoteric redox behavior, electron transfer and spectroscopic properties. Chemistry - A European Journal, 8(20), 4656-4669. https://doi.org/10.1002/1521-3765%2820021018%298%3A20%3C4656%3A%3Aaid-chem4656%3E3.0.co%3B2-1
- Functionalized pyridylboronic acids and their Suzuki cross-coupling reactions to yield novel heteroarylpyridinesParry, P., Wang, C., Batsanov, A., Bryce, M., & Tarbit, B. (2002). Functionalized pyridylboronic acids and their Suzuki cross-coupling reactions to yield novel heteroarylpyridines. Journal of Organic Chemistry, 67(21), 7541-7543.
- A blended layer MEH-PPV electroluminescent device incorporating a new electron transport materialCea, P., Hua, Y., Pearson, C., Wang, C., Bryce, M., Lopez, M., & Petty, M. (2002). A blended layer MEH-PPV electroluminescent device incorporating a new electron transport material. Materials Science and Engineering: C, 22(1), 87-89.
- An unexpected TTFAQ donor-fluorene acceptor reaction resulting in a novel salt: 2,6-dihexyloxy-9,10-bis(4,5-dimethyl-1,3-dithiol-2-ylium)-anthracene bis(2,5,7-trinitro-4-bromo-9-cyanofluorenide) dioxane trisolvateBatsanov, A., Bryce, M., Lyubchik, S., & Perepichka, I. (2002). An unexpected TTFAQ donor-fluorene acceptor reaction resulting in a novel salt: 2,6-dihexyloxy-9,10-bis(4,5-dimethyl-1,3-dithiol-2-ylium)-anthracene bis(2,5,7-trinitro-4-bromo-9-cyanofluorenide) dioxane trisolvate. Acta Crystallographica. Section B, Structural Science., 58, O1106-O1110.
- A (π-extended tetrathiafulvalene)-fluorene conjugate. Unusual electrochemistry and charge transfer properties: The first observation of a covalent D²⁺–σ–A˙⁻ redox statePerepichka, D., Bryce, M., Perepichka, I., Lyubchik, S., Christensen, C., Godbert, N., Batsanov, A., Levillain, E., McInnes, E., & Zhao, J. (2002). A (π-extended tetrathiafulvalene)-fluorene conjugate. Unusual electrochemistry and charge transfer properties: The first observation of a covalent D²⁺–σ–A˙⁻ redox state. Journal of the American Chemical Society, 124(47), 14227-14238. https://doi.org/10.1021/ja012518o
- Fluorescent phthalocyanine dimers - a steady state and flash photolysis studyFitzGerald, S., Farren, C., Stanley, C., Beeby, A., & Bryce, M. (2002). Fluorescent phthalocyanine dimers - a steady state and flash photolysis study. Photochemical & Photobiological Sciences, 1(8), 581-587.
- Synthesis of novel chiral bis(ferrocenyl) ligands and their use as voltammetric metal cation sensorsSutcliffe, O., Bryce, M., & Batsanov, A. (2002). Synthesis of novel chiral bis(ferrocenyl) ligands and their use as voltammetric metal cation sensors. Journal of Organometallic Chemistry, 656(1-2), 211-216.
- Photophysics of a fluorene co-polymer in solution and films.Palsson, L., Wang, C., Russell, D., Monkman, A., Bryce, M., Rumbles, G., & Samuel, I. (2002). Photophysics of a fluorene co-polymer in solution and films. Chemical Physics, 279(2-3), 229-237. https://doi.org/10.1016/s0301-0104%2802%2900407-x
- Protonation and subsequent intramolecular hydrogen bonding as a method to control chain structure and tune luminescence in heteroatomic conjugated polymersMonkman, A., Pålsson, L., Higgins, R., Wang, C., Bryce, M., Batsanov, A., & Howard, J. (2002). Protonation and subsequent intramolecular hydrogen bonding as a method to control chain structure and tune luminescence in heteroatomic conjugated polymers. Journal of the American Chemical Society, 124(21), 6049-6055. https://doi.org/10.1021/ja012409%2B
- Synthesis, structures and redox properties of tetrathiafulvalene and related bis(1,3-dithiole) dendrimersBryce, M., Nicholas, G., & Christensen, C. (2002). Synthesis, structures and redox properties of tetrathiafulvalene and related bis(1,3-dithiole) dendrimers. Abstracts of Papers: American Chemical Society Meetings, 223, D64-D64.
- Single layer polymer electroluminescent devices incorporating new electron transport materialsCea, P., Hua, Y., Pearson, C., Wang, C., Bryce, M., Royo, F., & Petty, M. (2002). Single layer polymer electroluminescent devices incorporating new electron transport materials. Thin Solid Films, 408(1-2), 275-281.
- New molecular electron-transport materials for light-emitting devicesBryce, M. (2002). New molecular electron-transport materials for light-emitting devices. Abstracts of Papers: American Chemical Society Meetings, 223, C22-C22.
- A biosensor for monitoring formaldehyde using a new lipophilic tetrathiafulvalene-tetracyanoquinodimethane salt and a polyurethane membraneKataky, R., Bryce, M., Goldenberg, L., Hayes, S., & Nowak, A. (2002). A biosensor for monitoring formaldehyde using a new lipophilic tetrathiafulvalene-tetracyanoquinodimethane salt and a polyurethane membrane. Talanta, 56(3), 451-458.
- Supramolecular architecture of two charge-transfer complexes based on 2,7-(X,X)-4,5-dinitro-9-dicyanomethylenefluorenes (X=NO2 or CN) and tetrathiafulvaleneKuz’mina, L., Perepichka, I., Perepichka, D., Howard, J., & Bryce, M. (2002). Supramolecular architecture of two charge-transfer complexes based on 2,7-(X,X)-4,5-dinitro-9-dicyanomethylenefluorenes (X=NO2 or CN) and tetrathiafulvalene. Crystallography Reports, 47(2), 251-261.
- The first genuine observation of fluorescent mononuclear phthalocyanine aggregatesFarren, C., FitzGerald, S., Beeby, A., & Bryce, M. (2002). The first genuine observation of fluorescent mononuclear phthalocyanine aggregates. Chemical Communications, 2002(6), 572-573. https://doi.org/10.1039/B110876J
- Synthesis, structure and optical characterisation of silicon phthalocyanine bis-estersFarren, C., FitzGerald, S., Bryce, M., Beeby, A., & Batsanov, A. (2002). Synthesis, structure and optical characterisation of silicon phthalocyanine bis-esters. Journal of the Chemical Society, Perkin Transactions 2, 2002(1), 59-66. https://doi.org/10.1039/B108778A
- New electron-transporting materials for light emitting diodes: 1,3,4-oxadiazole-pyridine and 1,3,4-oxadiazole-pyrimidine hybridsWang, C., Jung, G., Batsanov, A., Bryce, M., & Petty, M. (2002). New electron-transporting materials for light emitting diodes: 1,3,4-oxadiazole-pyridine and 1,3,4-oxadiazole-pyrimidine hybrids. Journal of Materials Chemistry, 12(2), 173-180.
- A stopperless tetrathiafulvalene based 2 pseudorotaxane molecular shuttle (vol 42, pg 4223, 2001)Bryce, M., Cooke, G., Devonport, W., Duclairoir, F., & Rotello, V. (2002). A stopperless tetrathiafulvalene based 2 pseudorotaxane molecular shuttle (vol 42, pg 4223, 2001). Tetrahedron Letters, 43(3), 537-537.
- New pi-electron rich donors and cavities and their supramolecular assemblies: Synthesis, electrochemistry and crystal structuresBryce, M., Batsanov, A., Godbert, N., & Christensen, C. (2002). New pi-electron rich donors and cavities and their supramolecular assemblies: Synthesis, electrochemistry and crystal structures. Molecular Crystals and Liquid Crystals, 379, 1-8.
- Voltammetric metal cation sensors based on ferrocene derivatives with oxazoline and imine substituentsSutcliffe, O., Chesney, A., & Bryce, M. (2001). Voltammetric metal cation sensors based on ferrocene derivatives with oxazoline and imine substituents. Journal of Organometallic Chemistry, 637, 134-138.
- Electron acceptors of the fluorene series. Part 12. 9- (Metalloceneylidene)nitrofluorene derivatives of Fc-NF, NF-Fc- NF, and NF-Rc-NF types, and the vinylogues Fc-pi-NF: synthesis, characterisation, intramolecular charge transfer, redox properties and X-Perepichka, D., Perepichka, I., Popov, A., Bryce, M., Batsanov, A., Chesney, A., Howard, J., & Sokolov, N. (2001). Electron acceptors of the fluorene series. Part 12. 9- (Metalloceneylidene)nitrofluorene derivatives of Fc-NF, NF-Fc- NF, and NF-Rc-NF types, and the vinylogues Fc-pi-NF: synthesis, characterisation, intramolecular charge transfer, redox properties and X-. Journal of Organometallic Chemistry, 637, 445-462.
- Cocrystals of 2-(2,4,5,7-tetranitrofluoren-9- ylidene)propanedinitrile and 2,4,5,7-tetranitrofluoren-9-one with chlorobenzeneBatsanov, A., Perepichka, I., Bryce, M., & Howard, J. (2001). Cocrystals of 2-(2,4,5,7-tetranitrofluoren-9- ylidene)propanedinitrile and 2,4,5,7-tetranitrofluoren-9-one with chlorobenzene. Acta Crystallographica C: Crystal Structure Communications, 57, 1299-1302.
- Electron acceptors of the fluorene series. Part 13. 9-(5- Nitrofuran-2-ylidene)- and 9-(5-nitro-2-thienylidene)-2,4,5,7- tetranitrofluorenes: novel pi-extended electron acceptors. Synthesis, cyclic voltammetry and X-ray crystal structures for the acceptorPerepichka, I., Perepichka, D., Lyubchik, S., Bryce, M., Batsanov, A., & Howard, J. (2001). Electron acceptors of the fluorene series. Part 13. 9-(5- Nitrofuran-2-ylidene)- and 9-(5-nitro-2-thienylidene)-2,4,5,7- tetranitrofluorenes: novel pi-extended electron acceptors. Synthesis, cyclic voltammetry and X-ray crystal structures for the acceptor. Journal of the Chemical Society, Perkin Transactions 2, 2001(9), 1546-1551.
- A stopperless tetrathiafulvalene based 2 pseudorotaxane molecular shuttleBryce, M., Cooke, G., Devonport, W., Duclairoir, F., & Rotello, V. (2001). A stopperless tetrathiafulvalene based 2 pseudorotaxane molecular shuttle. Tetrahedron Letters, 42(25), 4223-4226.
- Trialkyltetrathiafulvalene-sigma-tetracyanoanthraquinodimethane (R3TTF-sigma-TCNAQ) diads: Synthesis, intramolecular charge- transfer properties, and X-ray crystal structurePerepichka, D., Bryce, M., Batsanov, A., Howard, J., Cuello, A., Gray, M., & Rotello, V. (2001). Trialkyltetrathiafulvalene-sigma-tetracyanoanthraquinodimethane (R3TTF-sigma-TCNAQ) diads: Synthesis, intramolecular charge- transfer properties, and X-ray crystal structure. Journal of Organic Chemistry, 66(13), 4517-4524.
- Synthesis of conjugated tetrathiafulvalene (TTF)-pi-acceptor molecules - Intramolecular charge transfer and nonlinear optical propertiesBryce, M., Green, A., Moore, A., Perepichka, D., Batsanov, A., Howard, J., Ledoux-Rak, I., Gonzalez, M., Martin, N., Segura, J., Garin, J., Orduna, J., Alcala, R., & Villacampa, B. (2001). Synthesis of conjugated tetrathiafulvalene (TTF)-pi-acceptor molecules - Intramolecular charge transfer and nonlinear optical properties. European Journal of Organic Chemistry, 10, 1927-1935.
- The first tetrathiafulvalene-sigma-polynitrofluorene diads: Low HOMO-LUMO gap, amphoteric redox behavior, and charge transfer propertiesPerepichka, D., Bryce, M., McInnes, E., & Zhao, J. (2001). The first tetrathiafulvalene-sigma-polynitrofluorene diads: Low HOMO-LUMO gap, amphoteric redox behavior, and charge transfer properties. Organic Letters, 3(10), 1431-1434.
- Molecular saddles. 7. New 9,10-bis(1,3-dithiol-2-ylidene)-9,10- dihydroanthracene cyclophanes: Synthesis, redox properties, and X-ray crystal structures of neutral species and a dication saltChristensen, C., Batsanov, A., Bryce, M., & Howard, J. (2001). Molecular saddles. 7. New 9,10-bis(1,3-dithiol-2-ylidene)-9,10- dihydroanthracene cyclophanes: Synthesis, redox properties, and X-ray crystal structures of neutral species and a dication salt. Journal of Organic Chemistry, 66(10), 3313-3320.
- An efficient pyridine- and oxadiazole-containing hole-blocking material far organic light-emitting diodes: Synthesis, crystal structure, and device performanceWang, C., Jung, G., Hua, Y., Pearson, C., Bryce, M., Petty, M., Batsanov, A., Goeta, A., & Howard, J. (2001). An efficient pyridine- and oxadiazole-containing hole-blocking material far organic light-emitting diodes: Synthesis, crystal structure, and device performance. Chemistry of Materials, 13(4), 1167-1173.
- Synthesis and redox chemistry of tetrathiafulvalene and related bis(1,3-dithiole) dendrimersBryce, M., Christensen, C., & Godbert, N. (2001). Synthesis and redox chemistry of tetrathiafulvalene and related bis(1,3-dithiole) dendrimers. Abstracts of Papers: American Chemical Society Meetings, 221, 479-PMSE.
- Push-pull dithiole - fluorene accepters as electron transport materials for holographyPerepichka, D., Perepichka, I., Bryce, M., Moore, A., & Sokolov, N. (2001). Push-pull dithiole - fluorene accepters as electron transport materials for holography. Synthetic Metals, 121(1-3), 1487-1488.
- Photochemistry of the pi-extended 9,10-bis(1,3-dithiol-2- ylidene)-9,10-dihydroanthracene system: Generation and characterisation of the radical cation, dication, and derived productsJones, A., Christensen, C., Perepichka, D., Batsanov, A., Beeby, A., Low, P., Bryce, M., & Parker, A. (2001). Photochemistry of the pi-extended 9,10-bis(1,3-dithiol-2- ylidene)-9,10-dihydroanthracene system: Generation and characterisation of the radical cation, dication, and derived products. Chemistry - A European Journal, 7(5), 973-978. https://doi.org/10.1002/1521-3765%2820010302%297%3A5%3C973%3A%3Aaid-chem973%3E3.0.co%3B2-%23
- Fluorene co-polymer luminescence: implications for molecular interactions.Palsson, L., Wang, C., Monkman, A., Bryce, M., Rumbles, G., & Samuel, I. (2001). Fluorene co-polymer luminescence: implications for molecular interactions. Synthetic Metals, 119(1-3), 627-628.
- Spin-coated films of tetrakis-arborol-tetrathiafulvalenesLe Gall, T., Bryce, M., Moore, A., Pearson, C., & Petty, M. (2001). Spin-coated films of tetrakis-arborol-tetrathiafulvalenes. Synthetic Metals, 121(1-3), 1409-1410.
- Synthesis, crystal structure and electrochemistry of a new bimetallic nickel dithiolene complex (NBu4+)(2){C2S4 NiS2C2S2(CO) (2)}(2-). A precursor for molecular conductorsBatsanov, A., Bryce, M., Dhindsa, A., Howard, J., & Underhill, A. (2001). Synthesis, crystal structure and electrochemistry of a new bimetallic nickel dithiolene complex (NBu4+)(2){C2S4 NiS2C2S2(CO) (2)}(2-). A precursor for molecular conductors. Polyhedron, 20(6), 537-540.
- Convenient one pot synthesis of 5-unsubstituted pyrazolo 3,4- c isoquinolinesBogza, S., Kobrakov, K., Malienko, A., Sujkov, S., Perepichka, I., Bryce, M., Bogdan, N., & Dulenko, V. (2001). Convenient one pot synthesis of 5-unsubstituted pyrazolo 3,4- c isoquinolines. Journal of Heterocyclic Chemistry, 38(2), 523-525.
- Polymeric alkoxy PBD 2-(4-biphenylyl)-5-phenyl-1,3,4- oxadiazole for light-emitting diodesWang, C., Kilitziraki, M., Palsson, L., Bryce, M., Monkman, A., & Samuel, I. (2001). Polymeric alkoxy PBD 2-(4-biphenylyl)-5-phenyl-1,3,4- oxadiazole for light-emitting diodes. Advanced Functional Materials, 11(1), 47-50.
- Molecular saddles. 4. Redox-active cyclophanes by bridging the 9,10-bis(1,3-dithiol-2-ylidene)-9,10-dihydroanthracene system: Synthesis, electrochemistry, and X-ray crystal structures of neutral species and a dication saltGodbert, N., Batsanov, A., Bryce, M., & Howard, J. (2001). Molecular saddles. 4. Redox-active cyclophanes by bridging the 9,10-bis(1,3-dithiol-2-ylidene)-9,10-dihydroanthracene system: Synthesis, electrochemistry, and X-ray crystal structures of neutral species and a dication salt. Journal of Organic Chemistry, 66(3), 713-719.
- An investigation of the complexation behaviour of structurally modified tetrathiafulvalene derivatives with the electron deficient cyclophane cyclobis(paraquat-p-phenylene)Bryce, M., Cooke, G., Duclairoir, F., & Rotello, V. (2001). An investigation of the complexation behaviour of structurally modified tetrathiafulvalene derivatives with the electron deficient cyclophane cyclobis(paraquat-p-phenylene). Tetrahedron Letters, 42(6), 1143-1145.
- Hydroxymethyl-Functionalised 9,10-Bis(1,3-dithiol-2-ylidene)-9,10-Dihydroanthracene π-Electron Donors as Synthetic Intermediates for Supramolecular StructuresGodbert, N., Bryce, M., Dahaoui, S., Batsanov, A., Howard, J., & Hazendonk, P. (2001). Hydroxymethyl-Functionalised 9,10-Bis(1,3-dithiol-2-ylidene)-9,10-Dihydroanthracene π-Electron Donors as Synthetic Intermediates for Supramolecular Structures. European Journal of Organic Chemistry, 2001(4), 749-757. https://doi.org/10.1002/1099-0690%28200102%292001%3A4
- pi-Extended nitrofluorene-1,3-dithiole chromophore: enhancing the photoresponse of holographic materials through the balance of intramolecular charge transfer and electron affinityPerepichka, D., Perepichka, I., Bryce, M., Sokolov, N., & Moore, A. (2001). pi-Extended nitrofluorene-1,3-dithiole chromophore: enhancing the photoresponse of holographic materials through the balance of intramolecular charge transfer and electron affinity. Journal of Materials Chemistry, 11(7), 1772-1774.
- Synthesis and crystal engineering of new halogenated tetrathiafulvalene (TTF) derivatives and their charge transfer complexes and radical ion saltsBatsanov, A., Bryce, M., Chesney, A., Howard, J., John, D., Moore, A., Wood, C., Gershtenman, H., Becker, J., Khodorkovsky, V., Ellern, A., Bernstein, J., Perepichka, I., Rotello, V., Gray, M., & Cuello, A. (2001). Synthesis and crystal engineering of new halogenated tetrathiafulvalene (TTF) derivatives and their charge transfer complexes and radical ion salts. Journal of Materials Chemistry, 11(9), 2181-2191.
- Synthesis and Trapping of Transient 1,2-Diselones to Yield 1,4-Diselenin Derivatives: Calculated Structures of 1,2-Diselones, 1,2-Diselenetes and Their Sulfur AnaloguesBryce, M., Chesney, A., Yoshida, S., & Perepichka, I. (2000). Synthesis and Trapping of Transient 1,2-Diselones to Yield 1,4-Diselenin Derivatives: Calculated Structures of 1,2-Diselones, 1,2-Diselenetes and Their Sulfur Analogues. Chemistry - A European Journal, 6(1), 1-7.
- Crown-annelated 9,10-bis(1,3-dithiol-2-ylidene)-9,10-dihydroanthracene derivatives: a new efficient transducer in the electrochemical and spectroscopic monitoring of metal complexationBryce, M. R., Batsanov, A. S., Finn, T., & Hansen, T. K. (2000). Crown-annelated 9,10-bis(1,3-dithiol-2-ylidene)-9,10-dihydroanthracene derivatives: a new efficient transducer in the electrochemical and spectroscopic monitoring of metal complexation. Chemical Communications, 2000, 295-296. https://doi.org/10.1039/a908716h
- New 1,3-dithiol-2-ylidene donor-pi-acceptor chromophores with intramolecular charge-transfer properties, and related donor-pi-donor molecules: synthesis, electrochemistry, X-ray crystal structures, non-linear optical properties and theoretical calculationBryce, M., Moore, A., Batsanov, A., & Green, A. (1998). New 1,3-dithiol-2-ylidene donor-pi-acceptor chromophores with intramolecular charge-transfer properties, and related donor-pi-donor molecules: synthesis, electrochemistry, X-ray crystal structures, non-linear optical properties and theoretical calculation. Journal of Materials Chemistry, 8(5), 1173-1184.
- Synthesis of Pyrazinoporphyrazine Derivatives Functionalised with Tetrathiafulvalene (TTF) Units: X-Ray Crystal Structures of Two Related TTF Cyclophanes and Two Bis(1,3-dithiole-2-thione) IntermediatesBryce, M., Wang, C., Batsanov, A., & Howard, J. (1997). Synthesis of Pyrazinoporphyrazine Derivatives Functionalised with Tetrathiafulvalene (TTF) Units: X-Ray Crystal Structures of Two Related TTF Cyclophanes and Two Bis(1,3-dithiole-2-thione) Intermediates. Chemistry - A European Journal, 3(10), 1679-1690.
- An improved synthesis and structural characterisation of 2-(4-acetylthiophenylethynyl)-4-nitro-5-phenylethynylaniline: The molecule showing high negative differential resistance (NDR)Wang, C., Batsanov, A., Bryce, M., & Sage, I. (n.d.). An improved synthesis and structural characterisation of 2-(4-acetylthiophenylethynyl)-4-nitro-5-phenylethynylaniline: The molecule showing high negative differential resistance (NDR). Synthesis : Journal of Synthetic Organic Chemistry., 13, 2089-2095.
- Practical syntheses of N-hexylcarbazol-2-yl- and -3-yl-boronic acids, their cross-coupled products and a derived tris-cyclometalated (pyridin-2-yl)carbazole iridium(III) complexTavasli, M., Bettington, S., Bryce, M., Batsanov, A., & Monkman, A. (n.d.). Practical syntheses of N-hexylcarbazol-2-yl- and -3-yl-boronic acids, their cross-coupled products and a derived tris-cyclometalated (pyridin-2-yl)carbazole iridium(III) complex. Synthesis : Journal of Synthetic Organic Chemistry., 10, 1619-1624.
- Synthesis, structures and nonlinear optical properties of novel D-pi-A chromophores: Intramolecular charge transfer from 1,3- dithiole or ferrocene moieties to polynitrofluorene or dicyanomethylene moieties through conjugated linkersMoore, A., Chesney, A., Bryce, M., Batsanov, A., Kelly, J., Howard, J., Perepichka, I., Perepichka, D., Meshulam, G., Berkovic, G., Kotler, Z., Mazor, R., & Khodorkovsky, V. (n.d.). Synthesis, structures and nonlinear optical properties of novel D-pi-A chromophores: Intramolecular charge transfer from 1,3- dithiole or ferrocene moieties to polynitrofluorene or dicyanomethylene moieties through conjugated linkers. European Journal of Organic Chemistry, 14, 2671-2687.
- Synthesis of 2-heteroaryl-3-hydroxypyridines by ring expansion reactions of 2-acylfurans with ammoniaChubb, R., Bryce, M., & Tarbit, B. (n.d.). Synthesis of 2-heteroaryl-3-hydroxypyridines by ring expansion reactions of 2-acylfurans with ammonia. Journal of the Chemical Society, Perkin Transactions 1, 16, 1853-1854.
- Crown-annelated 9,10-bis(1,3-dithiol-2-ylidene)-9,10- dihydroanthracene derivatives as cation sensors: Synthesis, X- ray crystal structures, voltammetric and spectroscopic monitoring of metal complexationBryce, M., Batsanov, A., Finn, T., Hansen, T., Moore, A., Howard, J., Kamenjicki, M., Lednev, I., & Asher, S. (n.d.). Crown-annelated 9,10-bis(1,3-dithiol-2-ylidene)-9,10- dihydroanthracene derivatives as cation sensors: Synthesis, X- ray crystal structures, voltammetric and spectroscopic monitoring of metal complexation. European Journal of Organic Chemistry, 5, 933-940.
- Trimethyltetrathiafulvalene bearing an N-methylpyridinium substituent: Synthesis, crystal structures, and charge transfer propertiesMoore, A., Batsanov, A., Bryce, M., Howard, J., Khodorkovsky, V., Shapiro, L., & Shames, A. (n.d.). Trimethyltetrathiafulvalene bearing an N-methylpyridinium substituent: Synthesis, crystal structures, and charge transfer properties. European Journal of Organic Chemistry, 1, 73-78.

