Staff profile
Professor Gareth Williams
Professor

| Affiliation | Telephone |
|---|---|
| Professor in the Department of Chemistry | +44 (0) 191 33 42124 |
Biography
Prof J. A. Gareth Williams studied for an M.A. in Chemistry at Merton College, University of Oxford, including a year of research on the chemistry of dinuclear tungsten complexes in the group of Prof. Malcolm L. H. Green FRS. He then moved to the University of Durham to study for a PhD with Prof. David Parker FRS on the luminescence properties of macrocyclic metal complexes. That was followed by a postdoctoral fellowship at Universite Louis Pasteur, Strasbourg, with Prof. Jeane-Pierre Sauvage (Nobel Laureate 2016), working on the synthesis of multi-porphyin assemblies, and the study of energy- and electron-transfer processes within them, in collaboration with researchers at the C.N.R. Bologna, Italy.
Following further postdoctoral research, Williams was appointed to a lectureship at Durham University, promoted to Senior Lecturer, and then to full Professor in 2012.
He is author or co-author of around 240 publications, with an h index of 80 (Web of ScienceTM data, Jan 2025). Recent publications from the Group are listed at the end of this page. A full list can be viewed from the "ORCID profile" link above.
Research Interests
The Group's interests are centred around the synthesis and excited-state properties of light-emitting molecules ... particularly where there's a metal ion involved! We have a multi-disciplinary team, in which group members are typically engaged both in synthesis and optical spectroscopy. Applications of our molecular materials include: (i) emitters for organic light-emitting diodes (OLEDs) for new flat-screen display technology, (ii) luminescent probes for bioimaging and as sensors for bioactive molecules in solution, and (iii) photosensitisers of energy- and electron-transfer for solar energy conversion.
Synthetic work includes both organic synthesis and the coordination chemistry of transition metal and lanthanide ions. Luminescence and other photophysical properties are studied using steady-state and time-resolved absorption and emission spectroscopy in solution, in polymer films, and in solids, over a range of temperatures. The work is multi-disciplinary in nature and embraces all three of the main branches of chemistry. We have close links with Universities in France, Italy and North America, and industrial laboratories in the UK and USA.
We are also interested in the bacteriostatic effects of ligands related to EDTA - how such chelants can interfere with the ability of bacteria to acquire the metal ions that they need to survive. Such research has huge implications for the shelf-life of many consumer products, ranging from mayonnaise to face cream. We are exploring a bio-inspired approach to the problem of the environmental impact of the EDTA that is added to such products to preserve them. PhD student Lucy Woods leads the Group's current efforts in this area.
Cyclometallated platinum(II) and iridium(III) complexes
Organic light-emitting devices (OLEDs) are at the forefront of modern display screen technology. Luminescent, charge-neutral complexes of third-row transition metal ions like iridium and platinum function as "triplet-harvesting agents" in OLEDs. The high spin-orbit coupling constant of these heavy metal ions promotes emission from the triplet excited states that are normally non-emissive and otherwise wasted in such devices, leading to huge gains in efficiency and lower power consumption.
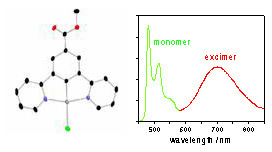
We have a particular interest in platinum(II) complexes. Being planar, they are able to undergo intermolecular face-to-face interactions that may lead to aggregates or excimers. These bimolecular excited states emit at lower energy than the isolated molecules, that is, further to the red and even into the near-infrared (NIR). We have been pioneering the use of such bimolecular states as a strategy to obtain efficient NIR OLEDs, and also for the red component that is needed to generate white light from an RGB display.
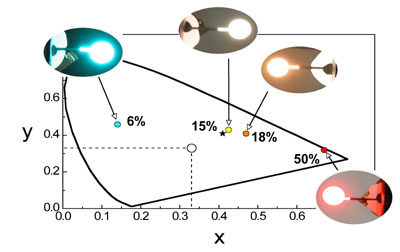
For example, the figure shows four OLEDs prepared using different concentrations of one of our platinum(II) complexes and the different colours that result, superimposed on the CIE coordinates. The asterisk indicates the ideal position for ambient room lighting (a bit redder than pure white) – and we’re not far off! The complex in this instance features a tridentate NCN-coordinating ligand based on 2,6-di(2-pyridyl)benzene, a structural type that we have developed extensively in our laboratory. We've discovered that these complexes are exceptionally good at forming brightly-emitting excimers and aggregates.
Meanwhile, we are also exploring the chemistry and excited states of platinum(IV) complexes with tridentate ligands. These are challenging to prepare due to the kinetic inertness of Pt(IV), but Yana Dikova, a PhD student in the group, has not been deterred!
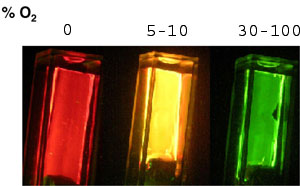
We have also investigated the utility of these compounds as oxygen sensors. By immobilising them in an ethyl cellulose film also containing platinum octaethylporphyrin, a wide-range O2 sensor is obtained that responds as a kind of molecular traffic light - as seen in the photograph above.
Luminescent sensors for bioactive ions and molecules in solution
Although a large number of fluorescent sensors for a variety of species are commercially available, most rely on changes in the wavelength or intensity of the short-lived (nanosecond) emission. We are seeking to develop new light-emitting components for sensors, in which the emission is long-lived, in the microsecond-to-millisecond range. This allows time-resolved detection methods of analysis to be employed, which gets round the problem of background interference from other fluorescent material, and also offers the potential for lifetime-based sensing.
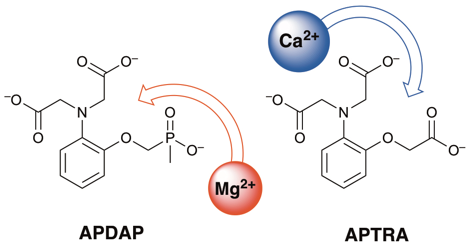
We're also interested in the challenging problem of designing fluorescent sensors for magnesium ions. There's been enormous progress over the past 30 years or so in the real-time detection of the biologically important metal ions Ca2+ and Zn2+. These metal ions can now be studied well in cellulo, using appropriate sensors in conjunction with fluorescence microscopy. But Mg2+ is difficult, because Ca2+ is usually a highly competitive ion: selectivity for Mg2+ is hard to achieve. We've found that by switching from the well-established ligand APTRA to a new phosphinate analogue APDAP (illustrated in the figure above), the affinity for Ca2+ is reduced far more than for Mg2+. The selectivity for Mg2+ is correspondingly enhanced greatly. This result offers a way to improved magnesium-selective fluorescent sensors. PhD student Laura Duncan is working on this challenging but rewarding topic.
Research interests
- Synthetic Chemistry
- Metal Complexes
- Luminescence and Bioimaging
Publications
Chapter in book
- Time-resolved emission imaging microscopy using phosphorescent metal complexes: taking FLIM and PLIM to new lengthsBaggaley, E., Weinstein, J., & Williams, J. (2014). Time-resolved emission imaging microscopy using phosphorescent metal complexes: taking FLIM and PLIM to new lengths. In K. Lo (Ed.), Luminescent and Photoactive Transition Metal Complexes as Biomolecular Probes and Cellular Reagents (pp. 205-256). Springer. https://doi.org/10.1007/430_2014_168
Journal Article
- Luminescent dinuclear platinum(II) complexes featuring rigidly linked Pt(N'CNO) units: N′ = isoquinoline versus pyridineSil, A., Puttock, E. V., & Williams, J. G. (2025). Luminescent dinuclear platinum(II) complexes featuring rigidly linked Pt(N’CNO) units: N′ = isoquinoline versus pyridine. Inorganica Chimica Acta, 587, Article 122823. https://doi.org/10.1016/j.ica.2025.122823
- In Pursuit of Low Energy Phosphorescence: Late Metal Coordination Complexes of the Planar, π‐extended Bipyridyl Ligand 6,6′,7,7′‐BiphenanthridineNemez, D. B., Ortiz, R. J., Veilleux, K. A., Williams, J. A. G., & Herbert, D. E. (2025). In Pursuit of Low Energy Phosphorescence: Late Metal Coordination Complexes of the Planar, π‐extended Bipyridyl Ligand 6,6′,7,7′‐Biphenanthridine. Chemistry – A European Journal. Advance online publication, Article e01802. https://doi.org/10.1002/chem.202501802
- 1,7-Dihalogenated BODIPYs: Synthesis, Structure and Photophysics.Nemez, D. B., Kacperkiewicz, A., Ortiz, R. J., Williams, J. A. G., & Herbert, D. E. (2025). 1,7-Dihalogenated BODIPYs: Synthesis, Structure and Photophysics. The Journal of Organic Chemistry. https://doi.org/10.1021/acs.joc.5c00407
- New members of the family of highly luminescent 1,3-bis(4-phenylpyridin-2-yl)-4,6-difluorobenzene platinum( ii ) complexes: exploring the effect of substituents on the 4-phenylpyridine unit †De Soricellis, G., Carboni, B., Guerchais, V., Williams, J. A. G., Marinotto, D., Colombo, A., Dragonetti, C., Fagnani, F., Fantacci, S., & Roberto, D. (2025). New members of the family of highly luminescent 1,3-bis(4-phenylpyridin-2-yl)-4,6-difluorobenzene platinum( ii ) complexes: exploring the effect of substituents on the 4-phenylpyridine unit †. Dalton Transactions. Advance online publication. https://doi.org/10.1039/d5dt00682a
- Enhanced Binding of Zn 2+ Using a Sulfur Version of o ‑Aminophenol-Triacetate (APTRA): Introducing S‑APTRA and DerivativesHogg, C., Duncan, L. L., Parker, D., & Williams, J. A. G. (2025). Enhanced Binding of Zn 2+ Using a Sulfur Version of o ‑Aminophenol-Triacetate (APTRA): Introducing S‑APTRA and Derivatives. Inorganic Chemistry, 64(19), 9509-9518. https://doi.org/10.1021/acs.inorgchem.5c00275
- Molecular Rectangles Featuring Two Parallel NCN ‐Coordinated Platinum Units: Enhancing Near‐Infrared Emission Through Excimer FormationSalthouse, R. J., Sil, A., Pander, P., Dias, F. B., & Williams, J. A. G. (2025). Molecular Rectangles Featuring Two Parallel NCN ‐Coordinated Platinum Units: Enhancing Near‐Infrared Emission Through Excimer Formation. Chemistry – A European Journal, 31(26), Article e202500834. https://doi.org/10.1002/chem.202500834
- Site-Selective Ligand Functionalization Reverses Hypsochromic Luminescence Shifts in Platinum(II) Complexes of Benzannulated NCN-Coordinating Ligands.Ortiz, R. J., Garcia-Torres, E., Brothwood, P. L., Williams, J. A. G., Herbert, D. E., & Brothwood, P. L. (2025). Site-Selective Ligand Functionalization Reverses Hypsochromic Luminescence Shifts in Platinum(II) Complexes of Benzannulated NCN-Coordinating Ligands. Chemistry - A European Journal, 31(6), Article e202403766. https://doi.org/10.1002/chem.202403766
- Dinuclear platinum( ii ) complexes emitting through TADF: new ligand design to minimise aggregation and the S 1 –T 1 energy gapPander, P., Dikova, Y. M., Puttock, E. V., & Williams, J. A. G. (2024). Dinuclear platinum( ii ) complexes emitting through TADF: new ligand design to minimise aggregation and the S 1 –T 1 energy gap. Inorganic Chemistry Frontiers, 21, 7191-7668. https://doi.org/10.1039/d4qi02069c
- Dinuclear platinum( ii ) complexes featuring rigidly linked Pt( NCN )X units: the effect of X = SCN − in favouring low-energy, excimer-like luminescenceSalthouse, R. J., Dikova, Y. M., Etherington, M. K., & Williams, J. A. G. (2024). Dinuclear platinum( ii ) complexes featuring rigidly linked Pt( NCN )X units: the effect of X = SCN − in favouring low-energy, excimer-like luminescence. New Journal of Chemistry, 48(44), 18865-18872. https://doi.org/10.1039/d4nj03357d
- Rigidly linked dinuclear platinum( ii ) complexes showing intense, excimer-like, near-infrared luminescencePander, P., Walden, M. T., Salthouse, R. J., Sil, A., Yufit, D. S., Dias, F. B., & Williams, J. A. G. (2023). Rigidly linked dinuclear platinum( ii ) complexes showing intense, excimer-like, near-infrared luminescence. Journal of Materials Chemistry C, 11(43), 15335-15346. https://doi.org/10.1039/d3tc03432a
- Cyclometallated Platinum(II) Complexes Featuring an Unusual, C^N‐Coordinating Pyridyl‐pyridylidene Ligand and L X Coligands: Synthesis, Structures and Dual Luminescence BehaviorMontagu, J., Gontard, G., Williams, J. A. G., & Moussa, J. (2023). Cyclometallated Platinum(II) Complexes Featuring an Unusual, C^N‐Coordinating Pyridyl‐pyridylidene Ligand and L X Coligands: Synthesis, Structures and Dual Luminescence Behavior. European Journal of Inorganic Chemistry, 26(32), Article e202300487. https://doi.org/10.1002/ejic.202300487
- Platinum(II) Complexes of Nonsymmetrical NCN-Coordinating Ligands: Unimolecular and Excimeric Luminescence Properties and Comparison with Symmetrical AnaloguesSalthouse, R. J., Sil, A., Gildea, L. F., Yufit, D. S., & Williams, J. A. G. (2023). Platinum(II) Complexes of Nonsymmetrical NCN-Coordinating Ligands: Unimolecular and Excimeric Luminescence Properties and Comparison with Symmetrical Analogues. Inorganic Chemistry, 62(31), 12356-12371. https://doi.org/10.1021/acs.inorgchem.3c01439
- Platinum(IV) Complexes with Tridentate, NNC-Coordinating Ligands: Synthesis, Structures, and LuminescenceDikova, Y. M., Yufit, D. S., & Williams, J. G. (2023). Platinum(IV) Complexes with Tridentate, NNC-Coordinating Ligands: Synthesis, Structures, and Luminescence. Inorganic Chemistry, 62(4), 1306-1322. https://doi.org/10.1021/acs.inorgchem.2c04116
- Enantiopure cycloplatinated pentahelicenic N-heterocyclic carbenic complexes that display long-lived circularly polarized phosphorescenceKundu, D., del Rio, N., Cordier, M., Vanthuyne, N., Puttock, E. V., Meskers, S. C. J., Williams, J. A. G., Srebro-Hooper, M., & Crassous, J. (2023). Enantiopure cycloplatinated pentahelicenic N-heterocyclic carbenic complexes that display long-lived circularly polarized phosphorescence. Dalton Transactions, 52(19), 6484-6493. https://doi.org/10.1039/d3dt00577a
- Modulation of chiroptical and photophysical properties in helicenic rhenium(I) systems: the use of an N‐(aza[6]helicenyl)‐NHC ligandGauthier, E. S., Abella, L., Caytan, E., Roisnel, T., Vanthuyne, N., Favereau, L., Srebro-Hooper, M., Williams, J. G., Autschbach, J., & Crassous, J. (2023). Modulation of chiroptical and photophysical properties in helicenic rhenium(I) systems: the use of an N‐(aza[6]helicenyl)‐NHC ligand. Chemistry – A European Journal, 29(21), Article 202203477. https://doi.org/10.1002/chem.202203477
- Yellow-Emitting, Pseudo-Octahedral Zinc Complexes of Benzannulated N^N^O Pincer-Type LigandsLozada, I. B., Braun, J. D., Williams, J. G., & Herbert, D. E. (2022). Yellow-Emitting, Pseudo-Octahedral Zinc Complexes of Benzannulated N^N^O Pincer-Type Ligands. Inorganic Chemistry, 61(44), 17568-17578. https://doi.org/10.1021/acs.inorgchem.2c02585
- Excimer or aggregate? Near infrared electro- and photoluminescence from multimolecular excited states of N^C^N-coordinated platinum(ii) complexesPander, P., Sil, A., Salthouse, R. J., Harris, C. W., Walden, M. T., Yufit, D. S., Williams, J. G., & Dias, F. B. (2022). Excimer or aggregate? Near infrared electro- and photoluminescence from multimolecular excited states of N^C^N-coordinated platinum(ii) complexes. Journal of Materials Chemistry C, 10(40), 15084-15095. https://doi.org/10.1039/d2tc01511k
- Helicenic N-heterocyclic carbene copper(I) complex displaying circularly polarized blue fluorescenceCrassous, J., Gauthier, E. S., Kaczmarczyk, D., Fré, S. D., Favereau, L., Caytan, E., Cordier, M., Vanthuyne, N., Williams, J. G., & Srebro-Hooper, M. (2022). Helicenic N-heterocyclic carbene copper(I) complex displaying circularly polarized blue fluorescence. Dalton Transactions, 51(40), 15571-15578. https://doi.org/10.1039/d2dt01925f
- Synthesis and Coordination Chemistry of a Benzannulated Bipyridine: 6,6′-BiphenanthridineNemez, D. B., Lozada, I. B., Braun, J. D., Williams, J. G., & Herbert, D. E. (2022). Synthesis and Coordination Chemistry of a Benzannulated Bipyridine: 6,6′-Biphenanthridine. Inorganic Chemistry, 61(34), 13386-13398. https://doi.org/10.1021/acs.inorgchem.2c01514
- Dual-emission luminescence thermometry using LaGaO3:Cr3+, Nd3+ phosphorsMullins, A. L., Ćirić, A., Zekovic, I., Williams, J. G., Dramicanin, M., & Evans, I. R. (2022). Dual-emission luminescence thermometry using LaGaO3:Cr3+, Nd3+ phosphors. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 10(28), 10396-10403. https://doi.org/10.1039/d2tc02011d
- Synthesis, Mesomorphism, Photophysics, and Device Properties of Liquid-Crystalline Pincer Complexes of Gold(III) Containing Semiperfluorinated ChainsParker, R. R., Stracey, R. F., McEllin, A. J., Chen, X., Wang, Y., Williams, J. G., Lynam, J. M., & Bruce, D. W. (2022). Synthesis, Mesomorphism, Photophysics, and Device Properties of Liquid-Crystalline Pincer Complexes of Gold(III) Containing Semiperfluorinated Chains. ACS Omega, 7(28), 24903-24917. https://doi.org/10.1021/acsomega.2c03669
- Double-deconvolution method for the separation of thermalised emissions from chromium-doped lanthanum gallate and its potential in luminescence-based thermometryMullins, A. L., Ćirić, A., Ristić, Z., Williams, J. G., Radosavljević Evans, I., & Dramićanin, M. D. (2022). Double-deconvolution method for the separation of thermalised emissions from chromium-doped lanthanum gallate and its potential in luminescence-based thermometry. Journal of Luminescence, 246. https://doi.org/10.1016/j.jlumin.2022.118847
- Luminescent bis-tridentate iridium(III) complexes: Overcoming the undesirable reactivity of trans-disposed metallated rings using –N^N^N–coordinating bis(1,2,4-triazolyl)pyridine ligandsWalden, M. T., Yufit, D. S., & Williams, J. G. (2022). Luminescent bis-tridentate iridium(III) complexes: Overcoming the undesirable reactivity of trans-disposed metallated rings using –N^N^N–coordinating bis(1,2,4-triazolyl)pyridine ligands. Inorganica Chimica Acta, 532, Article 120737. https://doi.org/10.1016/j.ica.2021.120737
- Insights into the antibacterial mechanism of action of chelating agents by selective deprivation of iron, manganese and zincPaterson, J. R., Beecroft, M. S., Mulla, R. S., Osman, D., Reeder, N. L., Caserta, J. A., Young, T. R., Pettigrew, C. A., Davies, G. E., Williams, J. G., & Sharples, G. J. (2022). Insights into the antibacterial mechanism of action of chelating agents by selective deprivation of iron, manganese and zinc. Applied and Environmental Microbiology, 88(2), Article e01641-21. https://doi.org/10.1128/aem.01641-21
- Platinum(ii) complexes of benzannulated N∧N−∧O-amido ligands: bright orange phosphors with long-lived excited statesLozada, I. B., Williams, J. G., & Herbert, D. E. (2022). Platinum(ii) complexes of benzannulated N∧N−∧O-amido ligands: bright orange phosphors with long-lived excited states. Inorganic Chemistry Frontiers, 9(1), 10-22. https://doi.org/10.1039/d1qi01120k
- Donor-Acceptor Boron-Ketoiminate Complexes with Pendent N-Heterocyclic Arms: Switched-on Luminescence through N-Heterocycle MethylationLozada, I. B., Ortiz, R. J., Braun, J. D., Williams, J. G., & Herbert, D. E. (2022). Donor-Acceptor Boron-Ketoiminate Complexes with Pendent N-Heterocyclic Arms: Switched-on Luminescence through N-Heterocycle Methylation. Journal of Organic Chemistry, 87(1), 184-196. https://doi.org/10.1021/acs.joc.1c02138
- Near-infrared electroluminescence beyond 940 nm in Pt(N^C^N)X complexes: influencing aggregation with the ancillary ligand XSalthouse, R. J., Pander, P., Yufit, D. S., Dias, F. B., & Williams, J. G. (2022). Near-infrared electroluminescence beyond 940 nm in Pt(N^C^N)X complexes: influencing aggregation with the ancillary ligand X. Chemical Science, 13(45), 13600-13610. https://doi.org/10.1039/d2sc05023d
- Brightly Luminescent Platinum Complexes of N∧C–∧N Ligands Forming Six-Membered Chelate Rings: Offsetting Deleterious Ring Size Effects Using Site-Selective BenzannulationOrtiz, R. J., Braun, J. D., Williams, J. G., & Herbert, D. E. (2021). Brightly Luminescent Platinum Complexes of N∧C–∧N Ligands Forming Six-Membered Chelate Rings: Offsetting Deleterious Ring Size Effects Using Site-Selective Benzannulation. Inorganic Chemistry, 60(22), 16881–16894. https://doi.org/10.1021/acs.inorgchem.1c02551
- Synthesis, structures and luminescence properties of N^C^N-coordinated platinum(II) complexes based on an anthracene core: a red shift and a twistWood, E. A., Gildea, L. F., Yufit, D. S., & Williams, J. G. (2021). Synthesis, structures and luminescence properties of N^C^N-coordinated platinum(II) complexes based on an anthracene core: a red shift and a twist. Polyhedron, 207, Article 115401. https://doi.org/10.1016/j.poly.2021.115401
- Helically chiral NHC‐gold(I) complexes: synthesis, chiroptical properties and electronic features of the [5]helicene‐imidazolylidene ligandGauthier, E. S., Cordier, M., Dorcet, V., Vanthuyne, N., Favereau, L., Williams, J. G., & Crassous, J. (2021). Helically chiral NHC‐gold(I) complexes: synthesis, chiroptical properties and electronic features of the [5]helicene‐imidazolylidene ligand. European Journal of Organic Chemistry, 2021(34), 4769-4776. https://doi.org/10.1002/ejoc.202100722
- The Role of Dinuclearity in Promoting Thermally Activated Delayed Fluorescence (TADF) in Cyclometallated, N^C^N-coordinated Platinum(II) ComplexesPander, P. H., Zaytsev, A., Sil, A., Williams, J. G., Lanoë, P., Kozhevnikov, V. N., & Dias, F. B. (2021). The Role of Dinuclearity in Promoting Thermally Activated Delayed Fluorescence (TADF) in Cyclometallated, N^C^N-coordinated Platinum(II) Complexes. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 9(32), 10276-10287. https://doi.org/10.1039/d1tc02562g
- Triskelion-shaped iridium-helicene NHC complexCrassous, J., Gauthier, E. S., Hellou, N., Caytan, E., Fré, S. D., Vanthuyne, N., Dorcet, V., Williams, G., Favereau, L., & Srebro-Hooper, M. (2021). Triskelion-shaped iridium-helicene NHC complex. Inorganic Chemistry Frontiers, 8(16), 3916-3925. https://doi.org/10.1039/d1qi00527h
- Enantioenriched Ruthenium-Tris-Bipyridine Complexes Bearing One Helical Bipyridine Ligand: Access to Fused Multihelicenic Systems and Chiroptical Redox SwitchesKos, M., Rodríguez, R., Storch, J., Sýkora, J., Caytan, E., Cordier, M., Císařová, I., Vanthuyne, N., Williams, J. G., Žádný, J., Církva, V., & Crassous, J. (2021). Enantioenriched Ruthenium-Tris-Bipyridine Complexes Bearing One Helical Bipyridine Ligand: Access to Fused Multihelicenic Systems and Chiroptical Redox Switches. Inorganic Chemistry, 60(16), 11838–11851. https://doi.org/10.1021/acs.inorgchem.1c01379
- Narrow-band red phosphors of high colour purity based on Eu3+-activated apatite-type Gd9.33(SiO4)6O2Rodriguez-Garcia, M. M., Ćirić, A., Ristić, Z., Williams, J. G., Dramicanin, M., & Evans, I. R. (2021). Narrow-band red phosphors of high colour purity based on Eu3+-activated apatite-type Gd9.33(SiO4)6O2. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 9(23), 7474-7484. https://doi.org/10.1039/d1tc01330k
- Designing magnesium-selective ligands using coordination chemistry principlesWalter, E. R., Hogg, C., Parker, D., & Gareth Williams, J. (2021). Designing magnesium-selective ligands using coordination chemistry principles. Coordination Chemistry Reviews, 428, Article 213622. https://doi.org/10.1016/j.ccr.2020.213622
- Platinum(II) Complexes of Tridentate ‐Coordinating Ligands Based on Imides, Amides, and Hydrazides: Synthesis and Luminescence PropertiesPuttock, E. V., Sturala, J., Kistemaker, J. C., & Williams, J. G. (2021). Platinum(II) Complexes of Tridentate ‐Coordinating Ligands Based on Imides, Amides, and Hydrazides: Synthesis and Luminescence Properties. European Journal of Inorganic Chemistry, 2021(4), 335-347. https://doi.org/10.1002/ejic.202000879
- Extended ligand conjugation and dinuclearity as a route to efficient platinum-based near-infrared (NIR) triplet emitters and solution-processed NIR-OLEDsShafikov, M. Z., Pander, P., Zaytsev, A. V., Daniels, R., Martinscroft, R., Dias, F. B., Williams, J. G., & Kozhevnikov, V. N. (2021). Extended ligand conjugation and dinuclearity as a route to efficient platinum-based near-infrared (NIR) triplet emitters and solution-processed NIR-OLEDs. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 9(1), 127-135. https://doi.org/10.1039/d0tc04881j
- Synthesis, Mesomorphism, Photophysics and Device Performance of Liquid-crystalline Pincer Complexes of Gold(III)Parker, R. R., Liu, D., Yu, X., Whitwood, A. C., Zhu, W., Williams, G., Wang, Y., Lynam, J. M., & Bruce, D. W. (2021). Synthesis, Mesomorphism, Photophysics and Device Performance of Liquid-crystalline Pincer Complexes of Gold(III). Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 2021(9), 1287-1302. https://doi.org/10.1039/d0tc04839a
- Synthesis, structure, photophysical and chiroptical properties of dinuclear rhenium complexes with a bridging helicene‐bis‐bipyridine ligandCrassous, J., Saleh, N., Kundu, D., Vanthuyne, N., Olesiak, J., Pniakowska, A., Matczyszyn, K., Chang, V., Muller, G., Williams, G., Srebro-Hooper, M., & Autschbach, J. (2020). Synthesis, structure, photophysical and chiroptical properties of dinuclear rhenium complexes with a bridging helicene‐bis‐bipyridine ligand. ChemPlusChem, 85(11), 2446-2454. https://doi.org/10.1002/cplu.202000559
- Deep-Red Luminescence from Platinum(II) Complexes of N^N–^N-Amido Ligands with Benzannulated N-Heterocyclic Donor ArmsMandapati, P., Braun, J. D., Lozada, I. B., Williams, J. G., & Herbert, D. E. (2020). Deep-Red Luminescence from Platinum(II) Complexes of N^N–^N-Amido Ligands with Benzannulated N-Heterocyclic Donor Arms. Inorganic Chemistry, 59(17), 12504-12517. https://doi.org/10.1021/acs.inorgchem.0c01584
- Mono and dinuclear iridium(iii) complexes featuring bis-tridentate coordination and Schiff-base bridging ligands: the beneficial effect of a second metal ion on luminescencePuttock, E. V., Sil, A., Yufit, D. S., & Williams, J. G. (2020). Mono and dinuclear iridium(iii) complexes featuring bis-tridentate coordination and Schiff-base bridging ligands: the beneficial effect of a second metal ion on luminescence. Dalton Transactions, 49(30), 10463-10476. https://doi.org/10.1039/d0dt01964j
- Long‐lived circularly‐polarized phosphorescence in helicene‐NHC‐rhenium(I) complexes: the influence of helicene, halogen and stereochemistry on emission propertiesCrassous, J., Gauthier, E. S., Abella, L., Hellou, N., Darquié, B., Caytan, E., Roisnel, T., Vanthuyne, N., Favereau, L., Srebro-Hooper, M., Williams, J. G., & Autschbach, J. (2020). Long‐lived circularly‐polarized phosphorescence in helicene‐NHC‐rhenium(I) complexes: the influence of helicene, halogen and stereochemistry on emission properties. Angewandte Chemie International Edition, 59(22), 8394-8400. https://doi.org/10.1002/anie.202002387
- Rotaxane Pt(II)-Complexes: Mechanical Bonding for Chemically Robust Luminophores and Stimuli Responsive BehaviourZhang, Z., Tizzard, G. J., Williams, J. G., & Goldup, S. (2020). Rotaxane Pt(II)-Complexes: Mechanical Bonding for Chemically Robust Luminophores and Stimuli Responsive Behaviour. Chemical Science, 7(11), 1839-1847. https://doi.org/10.1039/c9sc05507j
- Luminescent Platinum(II) Complexes of N^N–^N Amido Ligands with Benzannulated N-Heterocyclic Donor Arms: Quinolines Offer Unexpectedly Deeper Red Phosphorescence than PhenanthridinesMandapati, P., Braun, J. D., Killeen, C., Davis, R. L., Williams, J. G., & Herbert, D. E. (2019). Luminescent Platinum(II) Complexes of N^N–^N Amido Ligands with Benzannulated N-Heterocyclic Donor Arms: Quinolines Offer Unexpectedly Deeper Red Phosphorescence than Phenanthridines. Inorganic Chemistry, 58(21), 14808-14817. https://doi.org/10.1021/acs.inorgchem.9b02480
- A family of readily synthesised phosphorescent platinum(II) complexes based on tridentate N^N^O-coordinating Schiff-base ligandsPuttock, E., Fradgley, J., Yufit, D., & Williams, J. (2019). A family of readily synthesised phosphorescent platinum(II) complexes based on tridentate N^N^O-coordinating Schiff-base ligands. Dalton Transactions, 48(40), 15012-15028. https://doi.org/10.1039/c9dt03156a
- Fluorenylporphyrins functionalized by electrochromic ruthenium units as redox-triggered fluorescence switchesZhang, X., Abid, S., Shi, L., Williams, J. G., Fox, M. A., Miomandre, F., Tourbillon, C., Audibert, J., Mongin, O., Paul, F., & Paul-Roth, C. O. (2019). Fluorenylporphyrins functionalized by electrochromic ruthenium units as redox-triggered fluorescence switches. Dalton Transactions, 48(31), 11897-11911. https://doi.org/10.1039/c9dt02087j
- Single-Phase White-Emitting Phosphors Based on Apatite-Type Gadolinium Silicate, Gd9.33(SiO4)6O2 Doped with Dy3+, Eu3+ and Tb3+Rodriguez-Garcia, M. M., Williams, J. G., & Evans, I. R. (2019). Single-Phase White-Emitting Phosphors Based on Apatite-Type Gadolinium Silicate, Gd9.33(SiO4)6O2 Doped with Dy3+, Eu3+ and Tb3+. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 7(25), 7779-7787. https://doi.org/10.1039/c9tc02336d
- Homoleptic platinum(ii) complexes with pyridyltriazole ligands: excimer-forming phosphorescent emitters for solution-processed OLEDsWalden, M. T., Pander, P., Yufit, D. S., Dias, F. B., & Williams, J. G. (2019). Homoleptic platinum(ii) complexes with pyridyltriazole ligands: excimer-forming phosphorescent emitters for solution-processed OLEDs. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 7(22), 6592--6606. https://doi.org/10.1039/c9tc00768g
- A highly luminescent tetrahydrocurcumin Ir(III) complex with remarkable photoactivated anticancer activityDragonetti, C., Colombo, A., Fontani, M., Roberto, D., Williams, G., Scotto di Perrotolo, R., Casagrande, F., Barozzi, S., & Polo, S. (2019). A highly luminescent tetrahydrocurcumin Ir(III) complex with remarkable photoactivated anticancer activity. Chemistry - A European Journal, 25(33), 7948-7952. https://doi.org/10.1002/chem.201901527
- An enantiopure cyclometallated iridium complex displaying long-lived phosphorescence both in solution and in the solid stateCrassous, J., Macé, A., Hellou, N., Hammoud, J., Martin, C., Nasser, G., Gauthier, E., Favereau, L., Roisnel, T., Caytan, E., Vanthuyne, N., Williams, G., Berrée, F., & Carboni, B. (2019). An enantiopure cyclometallated iridium complex displaying long-lived phosphorescence both in solution and in the solid state. Helvetica Chimica Acta, 102(4). https://doi.org/10.1002/hlca.201900044
- Exploiting synergy between ligand design and counterion interactions to boost room temperature phosphorescence from Cu(i) compoundsMondal, R., Lozada, I. B., Davis, R. L., Williams, J. G., & Herbert, D. E. (2019). Exploiting synergy between ligand design and counterion interactions to boost room temperature phosphorescence from Cu(i) compounds. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 7(13), 3772-3778. https://doi.org/10.1039/c9tc00040b
- Dinuclear Design of a Pt(II) Complex Affording Highly Efficient Red Emission: Photophysical Properties and Application in Solution-Processible OLEDsShafikov, M. Z., Daniels, R., Pander, P., Dias, F. B., Williams, J. G., & Kozhevnikov, V. N. (2019). Dinuclear Design of a Pt(II) Complex Affording Highly Efficient Red Emission: Photophysical Properties and Application in Solution-Processible OLEDs. ACS Applied Materials and Interfaces, 11(8), 8182-8193. https://doi.org/10.1021/acsami.8b18928
- Quantification of energy transfer in bimetallic Pt(ii)–Ln(iii) complexes featuring an N^C^N-cyclometallating ligandEtchells, I. M., Pfrunder, M. C., Williams, J. G., & Moore, E. G. (2019). Quantification of energy transfer in bimetallic Pt(ii)–Ln(iii) complexes featuring an N^C^N-cyclometallating ligand. Dalton Transactions, 48(6), 2142-2149. https://doi.org/10.1039/c8dt04640a
- Synthesis, Mesomorphism, and Photophysics of 2,5-Bis(dodecyloxyphenyl)pyridine Complexes of Platinum(IV)Parker, R. R., Sarju, J. P., Whitwood, A. C., Williams, J. G., Lynam, J. M., & Bruce, D. W. (2018). Synthesis, Mesomorphism, and Photophysics of 2,5-Bis(dodecyloxyphenyl)pyridine Complexes of Platinum(IV). Chemistry - A European Journal, 24(71), 19010-19023. https://doi.org/10.1002/chem.201804026
- The luminescence properties of multinuclear platinum complexesPuttock, E. V., Walden, M. T., & Williams, J. G. (2018). The luminescence properties of multinuclear platinum complexes. Coordination Chemistry Reviews, 367, 127-162. https://doi.org/10.1016/j.ccr.2018.04.003
- APTRA-based luminescent lanthanide complexes displaying enhanced selectivity for Mg2+Parker, D., Walter, E., & Williams, J. (2018). APTRA-based luminescent lanthanide complexes displaying enhanced selectivity for Mg2+. Chemistry - A European Journal, 24(30), 7724-7733. https://doi.org/10.1002/chem.201800745
- On the antibacterial activity of azacarboxylate ligands: lowered metal ion affinities for bis-amide derivatives of EDTA do not mean reduced activityMulla, R. S., Beecroft, M. S., Pal, R., Aguilar, J., Pitarch-Jarque, J., García‐España, E., Lurie-Luke, E., Sharples, G., & Williams, J. (2018). On the antibacterial activity of azacarboxylate ligands: lowered metal ion affinities for bis-amide derivatives of EDTA do not mean reduced activity. Chemistry - A European Journal, 24(28), 7137-7148. https://doi.org/10.1002/chem.201800026
- Site-Selective Benzannulation of N-Heterocycles in Bidentate Ligands Leads to Blue-Shifted Emission from [(P^N)Cu]2(μ-X)2 DimersMondal, R., Lozada, I. B., Davis, R. L., Williams, J. G., & Herbert, D. E. (2018). Site-Selective Benzannulation of N-Heterocycles in Bidentate Ligands Leads to Blue-Shifted Emission from [(P^N)Cu]2(μ-X)2 Dimers. Inorganic Chemistry, 57(9), 4966-4978. https://doi.org/10.1021/acs.inorgchem.7b03223
- Tuning Mg2+ selectivity: comparative analysis of the photophysical properties of four fluorescent probes with an alkynyl-naphthalene fluorophoreParker, D., Williams, J., & Walter, E. (2018). Tuning Mg2+ selectivity: comparative analysis of the photophysical properties of four fluorescent probes with an alkynyl-naphthalene fluorophore. Chemistry - A European Journal, 24(24), 6432-6441. https://doi.org/10.1002/chem.201800013
- Enhanced selectivity for Mg2+ with a phosphinate-based chelate: APDAP versus APTRAWalter, E. R., Fox, M. A., Parker, D., & Williams, J. G. (2018). Enhanced selectivity for Mg2+ with a phosphinate-based chelate: APDAP versus APTRA. Dalton Transactions, 47(6), 1755-1763. https://doi.org/10.1039/c7dt04698g
- Solvent polarity and oxygen sensitivity, rather than viscosity, determine lifetimes of biaryl-sensitised terbium luminescenceWalter, E. R., Williams, J. G., & Parker, D. (2017). Solvent polarity and oxygen sensitivity, rather than viscosity, determine lifetimes of biaryl-sensitised terbium luminescence. Chemical Communications, 53(100), 13344-13347. https://doi.org/10.1039/c7cc08361k
- Photon Funnels for One-Way Energy Transfer: Multimetallic Assemblies Incorporating Cyclometallated Iridium or Rhodium Units Accessed by Sequential Cross-Coupling and BrominationKnuckey, K. J., & Williams, J. G. (2017). Photon Funnels for One-Way Energy Transfer: Multimetallic Assemblies Incorporating Cyclometallated Iridium or Rhodium Units Accessed by Sequential Cross-Coupling and Bromination. European Journal of Inorganic Chemistry, 2017(44), 5205-5214. https://doi.org/10.1002/ejic.201701020
- Monothiatruxene: a new versatile core for functional materialsMaciejczyk, M. R., Williams, J. G., Robertson, N., & Pietraszkiewicz, M. (2017). Monothiatruxene: a new versatile core for functional materials. RSC Advances, 7(78), 49532-49535. https://doi.org/10.1039/c7ra07671a
- Strategies for the synthesis of HBGl3, a glutamic acid derived ligand bearing phenolic and azacarboxylate donor groups at the nitrogen atomMulla, R. S., Walden, M. T., Yufit, D. S., Desa, T., Lurie-Luke, E., & Williams, J. G. (2017). Strategies for the synthesis of HBGl3, a glutamic acid derived ligand bearing phenolic and azacarboxylate donor groups at the nitrogen atom. Tetrahedron, 73(45), 6410-6420. https://doi.org/10.1016/j.tet.2017.09.032
- Monoamide Derivatives of EDTA Incorporating Pendent Carboxylates or Pyridyls: Synthesis, Metal Binding, and Crystal Structure of a Dinuclear Ca2+ Complex Featuring Bridging Na+ IonsMulla, R., Pitarch-Jarque, J., Garcia-Espana, E., Desa, T., Lurie-Luke, E., & Williams, J. (2017). Monoamide Derivatives of EDTA Incorporating Pendent Carboxylates or Pyridyls: Synthesis, Metal Binding, and Crystal Structure of a Dinuclear Ca2+ Complex Featuring Bridging Na+ Ions. ChemistrySelect, 2(18), 5045-5050. https://doi.org/10.1002/slct.201700995
- Rigidly linking cyclometallated Ir(III) and Pt(II) centres: an efficient approach to strongly absorbing and highly phosphorescent red emittersTurnbull, G., Williams, J., & Kozhevnikov, V. (2017). Rigidly linking cyclometallated Ir(III) and Pt(II) centres: an efficient approach to strongly absorbing and highly phosphorescent red emitters. Chemical Communications, 53(18), 2729-2732. https://doi.org/10.1039/c7cc00656j
- Tuning the dipolar second-order nonlinear optical properties of 5-π-delocalized-donor-1,3-di(2-pyridyl) benzenes, related cyclometallated platinum(II) complexes and methylated saltsNisic, F., Cariati, E., Colombo, A., Dragonetti, C., Fantacci, S., Garoni, E., Lucenti, E., Righetto, S., Roberto, D., & Williams, J. (2017). Tuning the dipolar second-order nonlinear optical properties of 5-π-delocalized-donor-1,3-di(2-pyridyl) benzenes, related cyclometallated platinum(II) complexes and methylated salts. Dalton Transactions, 46(4), 1179-1185. https://doi.org/10.1039/c6dt04359c
- Synthesis and Chiroptical Properties of Hexa-, Octa-, and Decaazaborahelicenes: Influence of Helicene Size and of the Number of Boron AtomsShen, C., Srebro-Hooper, M., Jean, M., Vanthuyne, N., Toupet, L., Williams, J., Torres, A., Riives, A., Muller, G., Autschbach, J., & Crassous, J. (2017). Synthesis and Chiroptical Properties of Hexa-, Octa-, and Decaazaborahelicenes: Influence of Helicene Size and of the Number of Boron Atoms. Chemistry - A European Journal, 23(2), 407-418. https://doi.org/10.1002/chem.201604398
- Metal Complexes for Two-Photon Photodynamic Therapy: A Cyclometallated Iridium Complex Induces Two-Photon Photosensitization of Cancer Cells under Near-IR LightMcKenzie, L., Sazanovich, I., Baggaley, E., Bonneau, M., Guerchais, V., Williams, J., Weinstein, J., & Bryant, H. (2017). Metal Complexes for Two-Photon Photodynamic Therapy: A Cyclometallated Iridium Complex Induces Two-Photon Photosensitization of Cancer Cells under Near-IR Light. Chemistry - A European Journal, 23(2), 234-238. https://doi.org/10.1002/chem.201604792
- New N^C^N-coordinated Pd(ii) and Pt(ii) complexes of a tridentate N-heterocyclic carbene ligand featuring a 6-membered central ring: synthesis, structures and luminescenceMoussa, J., Haddouche, K., Chamoreau, L., Amouri, H., & Williams, J. (2016). New N^C^N-coordinated Pd(ii) and Pt(ii) complexes of a tridentate N-heterocyclic carbene ligand featuring a 6-membered central ring: synthesis, structures and luminescence. Dalton Transactions, 45(32). https://doi.org/10.1039/c6dt02415g
- When two are better than one: bright phosphorescence from non-stereogenic dinuclear iridium(III) complexesDaniels, R., Culham, S., Hunter, M., Durrant, M., Probert, M., Clegg, W., Williams, J., & Kozhevnikov, V. (2016). When two are better than one: bright phosphorescence from non-stereogenic dinuclear iridium(III) complexes. Dalton Transactions, 45(16), 6949-6962. https://doi.org/10.1039/c6dt00881j
- Bimetallic Gold(I) Complexes with Ethynyl-Helicene and Bis-Phosphole Ligands: Understanding the Role of Aurophilic Interactions in their Chiroptical PropertiesEl Sayed Moussa, M., Chen, H., Wang, Z., Srebro-Hooper, M., Vanthuyne, N., Chevance, S., Roussel, C., Williams, J. G., Autschbach, J., Réau, R., Duan, Z., Lescop, C., & Crassous, J. (2016). Bimetallic Gold(I) Complexes with Ethynyl-Helicene and Bis-Phosphole Ligands: Understanding the Role of Aurophilic Interactions in their Chiroptical Properties. Chemistry - A European Journal, 22(17), 6075-6086. https://doi.org/10.1002/chem.201600126
- Pressure-induced variations of MLCT and ligand-centered luminescence spectra in square-planar platinum(II) complexesRodrigue-Witchel, A., Rochester, D. L., Zhao, S., Lavelle, K. B., Williams, J. G., Wang, S., Connick, W. B., & Reber, C. (2016). Pressure-induced variations of MLCT and ligand-centered luminescence spectra in square-planar platinum(II) complexes. Polyhedron, 108, 151-155. https://doi.org/10.1016/j.poly.2015.12.011
- Conformational changes and chiroptical switching of enantiopure bis-helicenic terpyridine upon Zn2+bindingIsla, H., Srebro-Hooper, M., Jean, M., Vanthuyne, N., Roisnel, T., Lunkley, J. L., Muller, G., Williams, J. G., Autschbach, J., & Crassous, J. (2016). Conformational changes and chiroptical switching of enantiopure bis-helicenic terpyridine upon Zn2+binding. Chemical Communications, 52(35), 5932-5935. https://doi.org/10.1039/c6cc01748g
- Synthesis and Luminescence Properties of Cycloplatinated Complexes with a Chelating N∧C Pyridine-Derived N-Heterocyclic Carbene - Influence of 2,4,6-Triphenylphosphinine versus TriphenylphosphineMoussa, J., Freeman, G. R., Williams, J. G., Chamoreau, L., Herson, P., & Amouri, H. (2016). Synthesis and Luminescence Properties of Cycloplatinated Complexes with a Chelating N∧C Pyridine-Derived N-Heterocyclic Carbene - Influence of 2,4,6-Triphenylphosphinine versus Triphenylphosphine. European Journal of Inorganic Chemistry, 2016(5). https://doi.org/10.1002/ejic.201500879
- A heterotrimetallic Ir(III), Au(III) and Pt(II) complex incorporating cyclometallating bi- and tridentate ligands : simultaneous emission from different luminescent metal centres leads to broad-band light emissionMuñoz-Rodríguez, R., Buñuel, E., Fuentes, N., Williams, J., & Cárdenas, D. (2015). A heterotrimetallic Ir(III), Au(III) and Pt(II) complex incorporating cyclometallating bi- and tridentate ligands : simultaneous emission from different luminescent metal centres leads to broad-band light emission. Dalton Transactions, 44(18), 8394-8405. https://doi.org/10.1039/c4dt02761b
- Acid/base-triggered switching of circularly polarized luminescence and electronic circular dichroism in organic and organometallic helicenesSaleh, S., Moore, B., Srebro, M., Vanthuyne, N., Toupet, L., Williams, J., Roussel, C., Deol, K., Muller, G., Autschbach, J., & Crassous, C. (2015). Acid/base-triggered switching of circularly polarized luminescence and electronic circular dichroism in organic and organometallic helicenes. Chemistry - A European Journal, 21(4), 1673-1681. https://doi.org/10.1002/chem.201405176
- An unprecedented cyclometallated platinum(II)complex incorporating a phosphinine co-ligand: synthesis and photoluminescence behaviourMoussa, J., Cheminel, T., Freeman, G., Chamoreau, L., Williams, J., & Amouri, H. (2014). An unprecedented cyclometallated platinum(II)complex incorporating a phosphinine co-ligand: synthesis and photoluminescence behaviour. Dalton Transactions, 43(22), 8162-8165. https://doi.org/10.1039/c4dt00589a
- Platinum(II) Complexes of N^C^N‑Coordinating 1,3-Bis(2-pyridyl)benzene Ligands: Thiolate Coligands Lead to Strong Red Luminescence from Charge-Transfer StatesTarran, W., Freeman, G., Murphy, L., Benham, A., Kataky, R., & Williams, J. (2014). Platinum(II) Complexes of N^C^N‑Coordinating 1,3-Bis(2-pyridyl)benzene Ligands: Thiolate Coligands Lead to Strong Red Luminescence from Charge-Transfer States. Inorganic Chemistry, 53(11), 5738-5749. https://doi.org/10.1021/ic500555w
- Straightforward access to mono- and biscycloplatinated helicenes displaying circularly polarized phosphorescence by using crystallization resolution methodsShen, C., Anger, E., Srebro, M., Vanthuyne, N., Deol, K., Jefferson, T., Muller, G., Williams, J., Toupet, L., Roussel, C., Autschbach, J., Réau, R., & Crassous, J. (2014). Straightforward access to mono- and biscycloplatinated helicenes displaying circularly polarized phosphorescence by using crystallization resolution methods. Chemical Science, 5(5), 1915-1927. https://doi.org/10.1039/c3sc53442a
- Long-lived metal complexes open up microsecond lifetime imaging microscopy under multiphoton excitation: from FLIM to PLIM and beyondBaggaley, E., Botchway, S. W., Haycock, J. W., Morris, H., Sazanovich, I. V., Williams, J. G., & Weinstein, J. A. (2013). Long-lived metal complexes open up microsecond lifetime imaging microscopy under multiphoton excitation: from FLIM to PLIM and beyond. Chemical Science, 5(3), 879-886. https://doi.org/10.1039/c3sc51875b
- Multifunctional and Reactive Enantiopure Organometallic Helicenes: Tuning Chiroptical Properties by Structural Variations of Mono- and Bis(platinahelicene)sAnger, E., Rudolph, M., Norel, L., Zrig, S., Shen, C., Vanthuyne, N., Toupet, L., Williams, J., Roussel, C., Autschbach, J., Crassous, J., & Réau, R. (2011). Multifunctional and Reactive Enantiopure Organometallic Helicenes: Tuning Chiroptical Properties by Structural Variations of Mono- and Bis(platinahelicene)s. Chemistry - A European Journal, 17(50), 14178-14198. https://doi.org/10.1002/chem.201101866
- Emissive Metallomesogens Based on 2-Phenylpyridine Complexes of Iridium(III)Santoro, A., Prokhorov, A., Kozhevnikov, V., Whitwood, A., Donnio, B., Williams, J., & Bruce, D. (2011). Emissive Metallomesogens Based on 2-Phenylpyridine Complexes of Iridium(III). Journal of the American Chemical Society, 133(14), 5248-5251. https://doi.org/10.1021/ja201245s
- Phosphorescence vs Fluorescence in Cyclometalated Platinum(II) and Iridium(III) Complexes of (Oligo) thienylpyridinesKozhevnikov, D., Kozhevnikov, V., Shafikov, M., Prokhorov, A., Bruce, D., & Williams, J. (2011). Phosphorescence vs Fluorescence in Cyclometalated Platinum(II) and Iridium(III) Complexes of (Oligo) thienylpyridines. Inorganic Chemistry, 50(8), 3804-3815. https://doi.org/10.1021/ic200210e
- The time domain in co-stained cell imaging: time-resolved emission imaging microscopy using a protonatable luminescent iridium complexMurphy, L., Congreve, A., Pålsson, L., & Williams, J. (2010). The time domain in co-stained cell imaging: time-resolved emission imaging microscopy using a protonatable luminescent iridium complex. Chemical Communications, 46(46), 8743-8745. https://doi.org/10.1039/c0cc03705b
- Evidence for electric field dependent dissociation of exciplexes in electron donor-acceptor organic solid filmsKalinowski, J., Cocchi, M., Virgili, D., Fattori, V., & Williams, J. (2006). Evidence for electric field dependent dissociation of exciplexes in electron donor-acceptor organic solid films. Chemical Physics Letters, 432(1-3), 110-115. https://doi.org/10.1016/j.cplett.2006.10.059
- An introduction to thiol redox proteins in the endoplasmic reticulum and a review of current electrochemical methods of detection of thiolsKruusma, J., Benham, A. M., Gareth Williams, J. A., & Kataky, R. (2006). An introduction to thiol redox proteins in the endoplasmic reticulum and a review of current electrochemical methods of detection of thiols. Analyst, 131(4), 459-473. https://doi.org/10.1039/b515874e
- Synthesis, structure, and photophysical properties of luminescent platinum(II) complexes containing cyclometalated 4-styryl-functionalized 2-phenylpyridine ligandsYin, B., Niemeyer, F., Williams, J., Jiang, J., Boucekkine, A., Toupet, L., Le Bozec, H., & Guerchais, V. (2006). Synthesis, structure, and photophysical properties of luminescent platinum(II) complexes containing cyclometalated 4-styryl-functionalized 2-phenylpyridine ligands. Inorganic Chemistry, 45(21), 8584-8596. https://doi.org/10.1021/ic0607282
- Luminescent Complexes of Iridium(III) Containing N∧C∧N-Coordinating Terdentate LigandsWilkinson, A. J., Puschman, H., Howard, J. A. K., Foster, C. E., & Williams, J. A. G. (2006). Luminescent Complexes of Iridium(III) Containing N∧C∧N-Coordinating Terdentate Ligands. Inorganic Chemistry, 45(21), 8685-8699. https://doi.org/10.1021/ic061172l
- Synthesis, structural studies, theoretical calculations, and linear and nonlinear optical properties of terpyridyl lanthanide complexes: New evidence for the contribution of f electrons to the NLO activitySénéchal-David, K., Hemeryck, A., Tancrez, N., Toupet, L., Williams, J., Ledoux, I., Zyss, J., Boucekkine, A., Guégan, J., Le Bozec., H., & Maury, O. (2006). Synthesis, structural studies, theoretical calculations, and linear and nonlinear optical properties of terpyridyl lanthanide complexes: New evidence for the contribution of f electrons to the NLO activity. Journal of the American Chemical Society, 128(37), 12243-12255. https://doi.org/10.1021/ja063586j
- Mutations in the FAD binding domain cause stress-induced misoxidation of the endoplasmic reticulum oxidoreductase Ero1bDias-Gunasekara, S., van Lith, M., Williams, J., Kataky, R., & Benham, A. (2006). Mutations in the FAD binding domain cause stress-induced misoxidation of the endoplasmic reticulum oxidoreductase Ero1b. Journal of Biological Chemistry, 281(35), 25018-25025. https://doi.org/10.1074/jbc.m602354200
- Synthesis and pH-sensitive luminescence of bis-terpyridyl iridium(III) complexes incorporating pendent pyridyl groups.Arm, K., Leslie, W., & Williams, J. (2006). Synthesis and pH-sensitive luminescence of bis-terpyridyl iridium(III) complexes incorporating pendent pyridyl groups. Inorganica Chimica Acta, 359(4), 1222-1232. https://doi.org/10.1016/j.ica.2005.09.021
- A novel luminescence-based colorimetric oxygen sensor with a "traffic light" response.Evans, R., Douglas, P., Williams, J., & Rochester, D. (2006). A novel luminescence-based colorimetric oxygen sensor with a "traffic light" response. Journal of Fluorescence, 16(2), 201-206. https://doi.org/10.1007/s10895-005-0037-9
- A cross-coupling strategy for the synthesis of dimetallic assemblies containing mixed bipyridine–terpyridine bridging ligands: luminescence and energy transfer properties.Arm, K., & Williams, J. (2006). A cross-coupling strategy for the synthesis of dimetallic assemblies containing mixed bipyridine–terpyridine bridging ligands: luminescence and energy transfer properties. Dalton Transactions, 2006(18), 2172-2174. https://doi.org/10.1039/b602022d
- Highly efficient exciplex phosphorescence from organic light-emitting diodesVirgili, D., Cocchi, M., Fattori, V., Sabatini, C., Kalinowski, J., & Williams, J. (2006). Highly efficient exciplex phosphorescence from organic light-emitting diodes. Chemical Physics Letters, 433(1-3), 145-149. https://doi.org/10.1016/j.cplett.2006.11.033
- Tissue-specific expression and dimerization of the endoplasmic reticulum oxidoreductase ErolbDias-Gunasekara, S., Gubbens, J., van Lith, M., Dunne, C., Williams, J., Kataky, R., Scoones, D., Lapthorn, A., Bulleid, N., & Benham, A. (2005). Tissue-specific expression and dimerization of the endoplasmic reticulum oxidoreductase Erolb. Journal of Biological Chemistry, 280(38), 33066-33075. https://doi.org/10.1074/jbc.m505023200
- Boronic acid-substituted metal complexes: versatile building blocks for the synthesis of multimetallic assembliesArm, K., & Williams, J. (2005). Boronic acid-substituted metal complexes: versatile building blocks for the synthesis of multimetallic assemblies. Chemical Communications, 2005(2), 230-232. https://doi.org/10.1039/b414929g
- Sensitised luminescence from phenanthridine appended lanthanide complexes: analysis of triplet mediated energy transfer processes in terbium, europium and neodymium complexesBeeby, A., Faulkner, S., Parker, D., & Williams, J. (2001). Sensitised luminescence from phenanthridine appended lanthanide complexes: analysis of triplet mediated energy transfer processes in terbium, europium and neodymium complexes. Journal of the Chemical Society, Perkin Transactions 2, 2001(8), 1268-1273. https://doi.org/10.1039/B009624P
- Chiroptical, ESMS and NMR spectroscopic study of the interaction of enantiopure lanthanide complexes with selected self-complementary dodecamer oligonucleotidesBobba, G., Dickins, R., Kean, S., Mathieu, C., Parker, D., Peacock, R., Siligardi, G., Smith, M., Williams, J., & Geraldes, C. (n.d.). Chiroptical, ESMS and NMR spectroscopic study of the interaction of enantiopure lanthanide complexes with selected self-complementary dodecamer oligonucleotides. Journal of the Chemical Society, Perkin Transactions 2, 9, 1729-1737.

