Staff profile
Overview
https://apps.dur.ac.uk/biography/image/1035
Dr Eckart Wrede
Associate Professor

| Affiliation | Telephone |
|---|---|
| Associate Professor in the Department of Chemistry | +44 (0) 191 33 42129 |
Biography
Research Interests
What happens during a chemical reaction? How do atoms move to break and form bonds? Why is one outcome more likely than another? These are some of the questions we seek to answer. Experimentally, we undertake very detailed measurements of reactive collisions between atoms and molecules. We use laser spectroscopic techniques to determine both the quantum state and the scattering angle of the products. Theoretically, we model these processes to reproduce and explain the experimental findings and to gain greater insight into their nature.
Reactive Scattering of Rydberg Atoms
Gas phase reactions between neutral reactants (A + BC → AB + C) or ions (e.g. A+ + BC → AB + C+) have been studied for many decades. A new and fascinating variation of this type of bimolecular reaction involves the use of highly excited Rydberg atoms, A*, as reactants: A* + BC → AB + C*. In a Rydberg atom, the highly excited Rydberg electron orbits around the ionic core at great distance and moves slowly compared to the nuclei. Thus, the Rydberg electron can be viewed as a spectator while the ionic reaction takes place. The size of the electron orbit can be controlled by the amount of Rydberg excitation. This allows the character of the reaction to be varied between its neutral (small orbit) and ionic (large orbit) 'extremes'. The aim of this project is to study this kind of reaction as a function of Rydberg excitation. Open and very interesting questions are: How does the reaction depend on the Rydberg state of the atom? Does the Rydberg state change during the reaction? The new experiment consists of a vacuum chamber with two molecular beams, a high power laser system and an ion imaging detector. This is an entirely new field of scattering dynamics with the potential for many interesting discoveries.Laser Spectroscopy in Molecular Beams
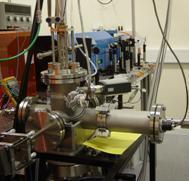 Spectroscopy, the interaction of light with matter, is a tool to determine the structure and internal motions (vibrations and rotations) of molecules. We employ a highly sensitive laser spectroscopic technique, called cavity ring-down spectroscopy, to examine specific vibrational motions of organic molecules in the gas phase. Spectra of large molecules taken at room temperature are often too crowded or even featureless due to the large number of excited internal motions. We reduce this internal excitation by cooling the molecules in a supersonic beam. The molecular beam intersects the laser and allows us to measure spectra of cold molecules which reveal the detail of individual vibrational modes.
Spectroscopy, the interaction of light with matter, is a tool to determine the structure and internal motions (vibrations and rotations) of molecules. We employ a highly sensitive laser spectroscopic technique, called cavity ring-down spectroscopy, to examine specific vibrational motions of organic molecules in the gas phase. Spectra of large molecules taken at room temperature are often too crowded or even featureless due to the large number of excited internal motions. We reduce this internal excitation by cooling the molecules in a supersonic beam. The molecular beam intersects the laser and allows us to measure spectra of cold molecules which reveal the detail of individual vibrational modes.Theoretical Studies of Reaction Mechanisms
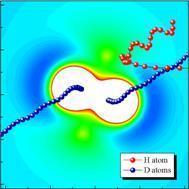 We model the motion of the atoms during a simple chemical reaction using classical mechanics. These quasi-classical trajectory calculations allow us to trace the motion of the atoms during the reaction and to identify different reaction mechanisms.
We have recently studied the hydrogen exchange reaction in its H + D2 → HD + D isotopic variant. This fundamental chemical reaction was thought to be well understood. However, our calculations have revealed new and unexpected reaction mechanisms. The figure to the left shows the motion of the three atoms during a reaction superimposed on the potential energy surface. In this particular collision, contrary to common thought, the H-atom does not attack the D2 molecule end-on (through the deep blue well) but impacts at the side near the so-called conical intersection (light green peak).
We model the motion of the atoms during a simple chemical reaction using classical mechanics. These quasi-classical trajectory calculations allow us to trace the motion of the atoms during the reaction and to identify different reaction mechanisms.
We have recently studied the hydrogen exchange reaction in its H + D2 → HD + D isotopic variant. This fundamental chemical reaction was thought to be well understood. However, our calculations have revealed new and unexpected reaction mechanisms. The figure to the left shows the motion of the three atoms during a reaction superimposed on the potential energy surface. In this particular collision, contrary to common thought, the H-atom does not attack the D2 molecule end-on (through the deep blue well) but impacts at the side near the so-called conical intersection (light green peak).
Cooling Molecules to Sub-Kelvin Temperatures
It is already possible to cool atoms to temperatures less than one millionth of a degree above absolute zero. At these temperatures, all the atoms in a sample drop into the same quantum state and form a Bose-Einstein condensate. The crucial next achievement will be to cool molecules to similar temperatures. Atomic Bose-Einstein condensation has already led to applications like lithography and precision measurement. Molecular condensates are vital for developments in quantum cryptography and quantum computing. They are also an important precursor to create new materials with specific quantum properties which can be used, e.g. to develop new types of sensors. We will employ different techniques to decelerate, cool and trap molecules. Once the temperature of the trapped molecules is characterised we plan to investigate the interactions between these ultra-cold molecules and atomic Bose-Einstein condensates.Selected Publications
-
Vibrational excitation through tug-of-war inelastic collisions.
S. J. Greaves, E. Wrede, N. T. Goldberg, J. Zhang, D. J. Miller, and R. N. Zare.
Nature 454 (2008), 88. -
New, unexpected and dominant mechanisms in the hydrogen exchange reaction.
S. J. Greaves, D. Murdock, E. Wrede, and S. C. Althorpe.
J. Chem. Phys. 128 (2008), 164306. -
On the dynamics of the H+ + D2(v=0, j=0) → HD + D+ reaction: A comparison between theory and experiment.
E. Carmona-Novillo, T. Gonzalez-Lezana, O. Roncero, P. Honvault, J.-M. Launay, N. Bulut, F. J. Aoiz, L. Bañares, A. Trottier, and E. Wrede.
J. Chem. Phys. 128 (2008), 014304. -
Cavity Ring-Down Spectroscopy of the Torsional Motions of 1,4-Bis(phenylethynyl)benzene.
S. J. Greaves, E. L. Flynn, E. L. Futcher, E. Wrede, D. P. Lydon, P. J. Low, S. R. Rutter, and A. Beeby.
J. Phys. Chem. A, 110 (2006), 2114. -
Theoretical Study of Geometric Phase Effects in the Hydrogen-Exchange Reaction.
J. C. Juanes-Marcos, S. C. Althorpe and E. Wrede.
Science 309 (2005), 1227. -
Reactive Scattering of Rydberg Atoms: H* + D2 → HD + D*.
E. Wrede, L. Schnieder, K. Seekamp-Schnieder, B. Niederjohann and K. H. Welge.
Phys. Chem. Chem. Phys. 7 (2005), 1577. -
Observation and interpretation of a time-delayed mechanism in the hydrogen-exchange reaction.
S. C. Althorpe, F. Fernández-Alonso, B. D. Bean, J. D. Ayers, A. E. Pomerantz, R. N. Zare, and E. Wrede.
Nature 416 (2002), 67. -
Continuum State Spectroscopy: A High Resolution Ion Imaging Study of IBr Photolysis in the Wavelength Range 440-685 nm.
E. Wrede, S. Laubach, S. Schulenburg, A. Brown, E. R. Wouters, A. J. Orr-Ewing, and M. N. R. Ashfold.
J. Chem. Phys. 114 (2001), 2629. -
Experimental determination of quantum state resolved differential cross sections for the hydrogen exchange reaction H + D2 → HD + D.
L. Schnieder, K. Seekamp-Rahn, E. Wrede, and K. H. Welge.
J. Chem. Phys. 107 (1997), 6175. -
Experimental Studies and Theoretical Predictions for the H + D2 → HD + D Reaction.
L. Schnieder, K. Seekamp-Rahn, J. Borkowski, E. Wrede, K. H. Welge, F. J. Aoiz, L. Bañares, M. J. D'Mello, V. J. Herrero, V. Sáez Rábanos, and R. E. Wyatt.
Science 269 (1995), 207-210.
Research interests
- Cold and Ultracold Molecules
- Gas Phase Molecular Reaction Dynamics
- Laser Spectroscopy
Esteem Indicators
- Secretary of the Spectroscopy and Dynamics Group of the RSC: Elected as secretary of the Spectroscopy and Dynamics Group (SDG) of the Faraday Division of the Royal Society of Chemistry (2008–2010).
Publications
Journal Article
- Absolute fluorescence and absorption measurements over a dynamic range of 106 with cavity-enhanced laser-induced fluorescenceSanders, S. E., Willis, O. R., Nahler, N. H., & Wrede, E. (2018). Absolute fluorescence and absorption measurements over a dynamic range of 106 with cavity-enhanced laser-induced fluorescence. Journal of Chemical Physics, 149(1), Article 014201. https://doi.org/10.1063/1.5031842
- Magnetic trapping of SH radicalsEardley, J., Warner, N., Deng, L., Carty, D., & Wrede, E. (2017). Magnetic trapping of SH radicals. Physical Chemistry Chemical Physics, 19(12), 8423-8427. https://doi.org/10.1039/c7cp00458c
- State purified deceleration of SD radicals by a Stark deceleratorNourbakhsh, O., Michan, J., Mittertreiner, T., Carty, D., Wrede, E., Djuricanin, P., & Momose, T. (2015). State purified deceleration of SD radicals by a Stark decelerator. Molecular Physics, 113(24), 4007-4018. https://doi.org/10.1080/00268976.2015.1109151
- Absolute density measurement of SD radicals in a supersonic jet at the quantum-noise-limitMizouri, A., Deng, L., Eardley, J., Nahler, N., Wrede, E., & Carty, D. (2013). Absolute density measurement of SD radicals in a supersonic jet at the quantum-noise-limit. Physical Chemistry Chemical Physics, 15(45), 19575-19579. https://doi.org/10.1039/c3cp53394h
- Production of cold bromine atoms at zero mean velocity by photodissociationDoherty, W., Bell, M., Softley, T., Rowland, A., Wrede, E., & Carty, D. (2011). Production of cold bromine atoms at zero mean velocity by photodissociation. Physical Chemistry Chemical Physics, 13(18), 8441-8447. https://doi.org/10.1039/c0cp02472d
- Photostop: production of zero-velocity molecules by photodissociation in a molecular beamTrottier, A., Carty, D., & Wrede, E. (2011). Photostop: production of zero-velocity molecules by photodissociation in a molecular beam. Molecular Physics, 109(5), 725-733. https://doi.org/10.1080/00268976.2010.550142
- Toward real-time charged-particle image reconstruction using polar onion-peelingRoberts, G., Nixon, J., Lecointre, J., Wrede, E., & Verlet, J. (2009). Toward real-time charged-particle image reconstruction using polar onion-peeling. Review of Scientific Instruments, 80(5), Article 053104. https://doi.org/10.1063/1.3126527
- Facile photoinduced charge separation through a cyanoacetylide bridge in a heterobimetallic Fe(ii)–Re(i) complexSmith, M. E., Flynn, E. L., Fox, M. A., Trottier, A., Wrede, E., Yufit, D. S., Howard, J. A., Ronayne, K. L., Towrie, M., Parker, A. W., Hartl, F., & Low, P. J. (2008). Facile photoinduced charge separation through a cyanoacetylide bridge in a heterobimetallic Fe(ii)–Re(i) complex. Chemical Communications, 2008(44), 5845-5847. https://doi.org/10.1039/b811357b
- Vibrational excitation through tug-of-war inelastic collisions.Greaves, S., Wrede, E., Goldberg, N., Zhang, J., Miller, D., & Zare, R. (2008). Vibrational excitation through tug-of-war inelastic collisions. Nature, 454(7200), 88-91. https://doi.org/10.1038/nature07079
- New, unexpected, and dominant mechanisms in the hydrogen exchange reactionGreaves, S., Murdock, D., Wrede, E., & Althorpe, S. (2008). New, unexpected, and dominant mechanisms in the hydrogen exchange reaction. Journal of Chemical Physics, 128(16), Article 164306. https://doi.org/10.1063/1.2902972
- Cavity ring-down spectroscopy of the torsional motions of 1,4-bis(phenylethynyl)benzeneGreaves, S., Flynn, E., Futcher, E., Wrede, E., Lydon, D., Low, P., Rutter, S., & Beeby, A. (2006). Cavity ring-down spectroscopy of the torsional motions of 1,4-bis(phenylethynyl)benzene. The Journal of Physical Chemistry A, 110(6), 2114-2121. https://doi.org/10.1021/jp054426h
- Theoretical study of geometric phase effects in the hydrogen-exchange reactionJuanes-Marcos, J., Althorpe, S., & Wrede, E. (2005). Theoretical study of geometric phase effects in the hydrogen-exchange reaction. Science, 309(5738), 1227-1230. https://doi.org/10.1126/science.1114890
- Observation and interpretation of a time-delayed mechanism in the hydrogen exchange reactionAlthorpe, S., Fernandez-Alonso, F., Bean, B., Ayers, J., Pomerantz, A., Zare, R., & Wrede, E. (2002). Observation and interpretation of a time-delayed mechanism in the hydrogen exchange reaction. Nature, 416(6876), 67-70. https://doi.org/10.1038/416067a

